Abstract
Background and Objective
Women with postpartum depression (PPD) may expose their infants to antidepressants via breast milk. Brexanolone is the only FDA-approved antidepressant specifically indicated for the treatment of PPD. This open-label, phase Ib study of healthy lactating volunteers assessed pharmacokinetic (PK) properties of brexanolone and a population PK (PopPK) model determined the relative infant dose (RID) in breastfeeding mothers.
Methods
Twelve participants received a 60-h infusion of brexanolone (titration up to 90 µg/kg/h). Allopregnanolone concentration was measured in breast milk and plasma. The RID was computed using a nonlinear mixed-effects PopPK model of patients with PPD and healthy women (N = 156). Model results were extended across an integrated dataset of participants through day 7.
Results
Allopregnanolone concentration–time profiles were similar between breast milk and plasma (partition coefficient for concentration gradient [milk : plasma] 1.36). Mean (95% CI) Cmax was 89.7 ng/mL (74.19–108.39), and median (95% CI) tmax was 47.8 h (47.8–55.8) in plasma. The overall PK profile was best described by a two-compartment model with linear elimination and distribution. Body weight was the only significant covariate identified. There were no apparent differences in PopPK AUC and Cmax between participants with or without concomitant antidepressant treatment. Maximum RID was 1.3%.
Conclusion
The PopPK model successfully described the variability and concentration–time profiles of allopregnanolone in breast milk and plasma in healthy participants and in the plasma of brexanolone-treated patients with PPD. The rapid elimination of allopregnanolone from plasma and breast milk, and low RID, suggests the appropriateness of brexanolone weight-based dosing and supports other PK-related labeling recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Given the potential infant exposure to antidepressants via excretion into breast milk, this study evaluated the concentrations of allopregnanolone in breast milk and maternal plasma from the start of a 60-h infusion of brexanolone injection through 7 days in 12 healthy volunteers. |
A population pharmacokinetic model was constructed to successfully describe the variability and concentration versus time profile of allopregnanolone in plasma of brexanolone-treated patients with postpartum depression (PPD) and healthy volunteers, and the distribution into breast milk of healthy volunteers (total, 156 women). The maximum relative infant dose was estimated to be 1.3% of the maternal dose. |
Together, these findings suggest the appropriateness of weight-based dosing and support other pharmacokinetic-related labeling recommendations of brexanolone in adult patients with PPD. |
1 Introduction
Postpartum depression (PPD), defined as a major depressive episode with onset during pregnancy or within the first 4 weeks postpartum, remains one of the most common medical complications during and after pregnancy [1,2,3]. In the United States, estimates of new mothers experiencing PPD symptoms vary by state from 9.7 to 23.5% annually (overall prevalence, 13.2%) [4]. Symptoms of PPD can be associated with significant impairment in mother–infant bonding and maternal function [5, 6], including breastfeeding [7] and reduced nurturing interactions or caring for the child [6, 7], all of which have implications for the child’s health and development [8,9,10,11]. Multiple environmental and biological risk factors have been proposed to play a role in the development of PPD, including a history of depression [3, 12].
Women at risk of peripartum depression have an altered neuroactive steroid profile and significantly lower peripartum γ-aminobutyric acid (GABA) levels compared with healthy women without PPD [13]. Allopregnanolone is an endogenous neuroactive steroid that functions as a GABA type A receptor (GABAAR) positive allosteric modulator [14, 15]. A large prospective study reported that increased allopregnanolone levels were significantly associated with the severity of peripartum depression and dysregulated excitatory–inhibitory balance in brain networks of women with PPD [16]. Allopregnanolone levels have been shown to vary during the menstrual cycle from 0.5 nmol/L in the follicular phase to approximately 4–5 nmol/L in the mid-luteal phase [17]. During pregnancy, allopregnanolone levels rise almost 10-fold from the maximum menstrual cycle levels, while the GABAAR are downregulated; however, allopregnanolone levels rapidly drop to approximately 2 nmol/L (0.637 ng/mL) following childbirth, while the levels of the GABAAR subunit are upregulated [13, 17]. Failure of GABAAR to adapt to decreased allopregnanolone levels at parturition in preclinical studies in mice has been suggested to play a role in the development of PPD [18].
Brexanolone is a synthetic analog of allopregnanolone [14, 15]. Unlike benzodiazepines, which target only synaptic GABAAR, brexanolone targets both synaptic and extrasynaptic GABAAR [19, 20]. Brexanolone selectively interacts with GABAAR, which may help restore excitatory–inhibitory balance in the brain [14, 18]. The efficacy and safety of brexanolone were demonstrated in three randomized, double-blind, placebo-controlled trials and in one open-label proof-of-concept study in women with PPD [14, 15, 21]. These studies showed that a single 60-h infusion of brexanolone titrated to a maximum dosage of 90 μg/kg/h (BRX90) resulted in rapid and statistically significant improvements in depressive symptoms compared with placebo, with acceptable tolerability. Furthermore, the BRX90 dose regimen yielded plasma concentrations of allopregnanolone that approximated endogenous levels at the end of pregnancy [22]. Brexanolone, as a 60-h intravenous infusion, was approved in 2019 by the US Food and Drug Administration (FDA) for the treatment of adults with PPD [23].
Approximately 2.9–5.5% of nursing mothers have been reported using antidepressants in European countries [24, 25]. Pregnancy-related physiological changes and breastfeeding may impact the pharmacokinetics (PK) of antidepressants, and these changes have been linked to higher dose requirements to maintain a therapeutic antidepressant dose during pregnancy, especially during the third trimester [26, 27]. The ability of an antidepressant to enter breast milk is dependent on several factors, including its protein binding, absorption rate, half-life, volume of distribution, and solubility [28, 29]. In addition, milk-related factors (pH, protein content, and lipid content) impact the drug’s ability to enter breast milk [27]. However, conventional PK studies in postpartum breastfeeding mothers present practical and ethical constraints, making it difficult to examine interindividual variations of drug excretion into breast milk and infant exposure [28, 29]. There is a general lack of data from large-scale outcome trials describing antidepressant exposure to the infant through breast milk that may contribute to premature discontinuation of breastfeeding or poor adherence by the mother to drug therapy [28]. Population PK (PopPK) studies are based on sparse sampling from a relatively large population and are ideal for simulation analyses of variations of drug exposure levels in breastfeeding mothers and their infants [28, 29].
Given the potential infant exposure to antidepressants via excretion into breast milk, this study aimed to evaluate the concentrations of allopregnanolone in breast milk and plasma of healthy lactating volunteers from the start of a 60-h infusion of BRX90 through 7 days. To further characterize maternal milk and plasma concentrations of allopregnanolone in lactating women, a PopPK model was constructed to assess the impact of clinically relevant covariates on the PK of BRX90 in an integrated dataset of brexanolone-treated patients with PPD. The model was used to predict the brexanolone dose exposure of the infant through simulation and to calculate the relative infant dose (RID).
2 Methods
2.1 Study Participants
This was an open-label, phase Ib lactation study designed to assess the concentration of allopregnanolone in the breast milk of healthy adult lactating women during and after brexanolone intravenous infusion. The study enrolled 12 non-smoking participants aged 18–45 years who were ≤ 6 months postpartum and lactating. Participants agreed to temporarily discontinue breastfeeding until day 7 after initiation of brexanolone infusion. Participants agreed to maximally pump breast milk (at least every 12 h) from day 1 until day 7. Participants with renal failure, fulminant hepatic failure, anemia, known allergy to progesterone or allopregnanolone, or exposure to other investigational study drugs or devices within 30 days prior to screening were excluded.
The study was conducted at six clinical sites in the United States. The study protocol was reviewed and approved by an Independent Ethics Committee and/or Institutional Review Board (Schulman Associates Institutional Review Board, Inc; Cincinnati, OH, USA), and the study was conducted in accordance with the Declaration of Helsinki, the International Conference for Harmonisation, and Good Clinical Practice guidelines. Written informed consent was obtained from all participants before starting any study procedure.
2.2 Study Design
Participants received a single 60-h continuous infusion of brexanolone, with a dosing regimen as follows: 30 μg/kg/h (BRX30; 0–4 h), 60 μg/kg/h (BRX60; 4–24 h), BRX90 (24–52 h), BRX60 (52–56 h), and BRX30 (56–60 h) [23]. Participants were discharged after completion of hour 72 safety assessments (see below) and were followed up with an outpatient visit on day 7. Blood samples for analysis of plasma allopregnanolone were collected pre-infusion at day 1, at pre-defined time points (hours 12, 24, 36, 48, 56, 60, 61, 62, and 64) until 72 h after the start of infusion, and at day 7. PK blood draws during the treatment period had a window of ± 10 min. In the event of an unplanned dose adjustment, the unscheduled PK sample was collected just prior to the infusion rate change. Breast milk samples were collected pre-infusion; at least every 12 h between hour 0 and hour 72; and at days 4, 5, 6, and 7. The collected plasma and breast milk samples were kept frozen until analyzed.
The primary endpoint was allopregnanolone levels in breast milk after a 60-h infusion of brexanolone in healthy lactating women. Secondary endpoints were allopregnanolone levels in plasma after a 60-h brexanolone infusion and the safety and tolerability of a 60-h brexanolone infusion in healthy lactating women.
2.3 Assessments
2.3.1 Pharmacokinetics
Plasma samples were collected for PK analyses at predefined time points. Breast milk samples were collected ad libitum and retained for analysis. Allopregnanolone levels in plasma and breast milk were determined using a validated high-performance liquid chromatography–mass spectroscopy (LC–MS) method. The lower limit of quantitation (LLOQ) was 1 ng/mL for plasma and 5 ng/mL for breast milk. Assay methods were documented internally and calibrated to detect allopregnanolone levels during infusion conditions rather than detection of low endogenous levels. Pharmacokinetic parameters derived from plasma brexanolone concentrations by model-independent methods (non-compartmental analysis) included area under the concentration–time curve (AUC) from time 0–60 h (AUC0–60), AUC from time 0–infinity (AUC0–∞), AUC from time 24–56 h (AUC24–56) during the infusion plateau, maximum plasma drug concentration (Cmax), time to peak plasma concentration (tmax), steady-state drug concentration in plasma during constant-rate infusion (Css), clearance (CL), and average drug concentration in plasma at steady state during the infusion plateau (Cavg). Breast milk volume, allopregnanolone concentration (ng/mL), and amount of allopregnanolone (µg) were determined for each sample.
2.3.2 Safety
Safety was assessed by adverse events (AEs), vital signs, clinical laboratory measurements, electrocardiograms, and suicidal ideation and behavior (via Columbia Suicide Severity Rating Scale) [30]. All AEs were coded using the Medical Dictionary for Regulatory Activities Version 20.0. Treatment-emergent AEs (TEAEs; defined as AEs that occurred after treatment initiation) and serious AEs (SAEs) were recorded through day 7. Vital signs were measured at screening; pre-infusion; and at h 2, 4, 8, 12, 18, 24, 30, 36, 42, 48, 56, 60, 66, 72, and day 7. Clinical laboratory assessments were performed at screening, hour 72, and day 7. Electrocardiogram and C-SSRS were assessed at screening, pre-infusion, hour 72, and day 7.
2.3.3 Population Pharmacokinetics (PopPK) Modeling
Data from three randomized, double-blind, placebo-controlled trials (ClinicalTrials.gov identifiers: NCT02614547, NCT02942004, NCT02942017) [14, 15]; one open-label study (NCT02285504) [21]; and one open-label study of healthy lactating women (547-CLP-108; current study; see Electronic Supplementary Materials [ESM]) were used to evaluate the PopPK properties of brexanolone. Patients with PPD in the three controlled trials and healthy lactating women in the current study received a 60-h continuous intravenous infusion of brexanolone, with infusion rates escalating up either to BRX60 by hour 4 or to BRX90 by hour 24. Brexanolone was tapered during the last 8 h of the infusion. Four patients with PPD in the open-label study received a slightly different schedule of a single 60-h continuous infusion of BRX90 (see ESM). Plasma samples were collected for PK analyses at predefined time points and were assayed by an LC–MS assay with an LLOQ of 1 ng/mL.
A PopPK model describing brexanolone PK in plasma and breast milk was developed using the current study and supported by phase II/III data (N = 156; see ESM for details on study characteristics, PK sampling, and model development). PopPK modeling analyses were conducted using nonlinear mixed-effects modeling (NONMEM®, Version 7.3, ICON Development Solutions, Ellicott City, MD, USA; see ESM). Models of increasing complexity were examined with NONMEM and were diagnosed and qualified using criteria for graphical assessment of goodness-of-fit, visual predictive check, shrinkage, numerical assessment of goodness-of-fit, and assessment of uncertainty-of-parameter estimates. The general form of the model was a two-compartment model for plasma with estimation of a partition coefficient to account for the concentration gradient between plasma and breast milk. Body weight was included in the base PopPK model prior to stepwise covariate modeling. Later, with availability of data from the full set of clinical trials, the model for plasma PK was updated to incorporate all available results while keeping constant the estimates for milk obtained based on 547-CLP-108. See ESM for full details.
2.4 Statistical Analysis
The safety set of the 547-CLP-108 study comprised all participants who were administered the study drug. The PK set comprised all participants with at least one evaluable PK sample. The breast milk set comprised all participants who began the infusion and had at least one breast milk sample collected. Continuous variables were summarized descriptively by number, mean, standard deviation, median, minimum, and maximum. Change from baseline values were calculated at each time point and summarized descriptively. Categorical variables were summarized with counts and percentages. Breast milk concentration of allopregnanolone, volume, and amount secreted were summarized by descriptive summary statistics, and are displayed in figures using both linear and log scales. Using the model parameters and assuming an infant feeding rate of 150 mL/kg/day [31], RID was computed as the infant dose divided by maternal dose, as previously described [32]. To test the hypothesis that concentrations in milk are a constant fraction of the plasma concentration and to support simulations in a more expansive population of patients, the model was used to characterize the shape of the milk concentration versus the time profile and to elucidate how long detectable levels of allopregnanolone might remain in the milk after cessation of dosing or at the end, after the completion of therapy.
where Dose.infant = dose in infant/day and Dose.mother = dose in mother/day.
3 Results
3.1 Participant Disposition and Demographics
All 12 healthy lactating women enrolled in this trial completed the study. The median age was 28.5 years (range 22–42 years), mean body weight was 83 kg, and a majority (75%) were White. Demographic and baseline characteristics of participants included in the PopPK model are described in Table 1.
3.2 Pharmacokinetic Properties
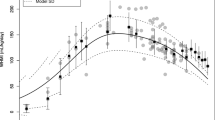
All pre-dose plasma samples were below the LLOQ for allopregnanolone (1 ng/mL). During the 60-h infusion, the mean plasma concentration of allopregnanolone increased with dose and subsequently decreased during dose taper (Fig. 1) [14, 15]. By 72 h, plasma allopregnanolone concentrations were approaching the LLOQ for all participants. All participants were near or below LLOQ by day 7 (Fig. 1).
Mean allopregnanolone concentration in plasma. Mean and standard error allopregnanolone plasma concentration by nominal time points for blood sample collection pre-infusion and at 12, 24 (before infusion-rate change), 36, 48, 56, 60 (before infusion end), 61, 62, 64, and 72 h after the start of infusion. Samples were also collected on day 7. All participants were near or below the lower limit of quantitation (LLOQ) (1 ng/mL) by day 7 after starting brexanolone infusion
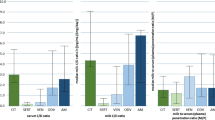
Variability in milk production between participants was relatively large but generally consistent for each individual. Because breast milk collection times varied by participant and pumping was ad libitum, mean concentration of allopregnanolone over time in breast milk could not be directly compared with mean plasma concentration over time, and the data were summarized individually. Allopregnanolone concentrations in breast milk declined rapidly after the end of the 60-h infusion to undetectable levels 3 days after the end of infusion (Fig. 2). This decline paralleled that observed in plasma allopregnanolone levels (Fig. 1). Maximum concentrations of allopregnanolone in breast milk were observed between hour 24 and hour 48, which corresponded with the administration of the maximum dosage in this period (Fig. 2). Concentrations of allopregnanolone in breast milk followed a temporal pattern similar to those in plasma, with the mean concentrations in breast milk approaching the LLOQ (5 ng/mL) on day 4 (11.6 ng/mL) and day 5 (5.6 ng/mL) (Table 2). Six of 11 participants on day 5 and 11 of 11 participants on day 6 had breast milk levels of allopregnanolone below the LLOQ.
Allopregnanolone concentration in breast milk for each participant. Breast milk was collected pre-infusion, at least every 12 h between hour 0 and hour 72, and on days 4, 5, 6, and 7. When possible, expression of breast milk, including the pre-infusion sample, occurred within ± 1 h of the collection of blood samples for PK analysis. Breast milk concentration data are presented for individual participants, as pumping was ad libitum. Maximum allopregnanolone concentration in breast milk was observed between hour 24 and hour 48, when the maximum brexanolone dose was being administered. PK pharmacokinetics
One of 12 participants in the PK set was excluded from the PK statistical analysis owing to discontinuation of dosing at hour 47. However, all available data were included in the PopPK model. A summary of the plasma PK parameters in the 11 participants analyzed is given in Table 3. The geometric mean (95% CI) Cmax was 89.7 ng/mL (74.2–108.4), tmax was 47.8 h (47.8–55.8), AUC0-60 was 3358.4 ng∙h/mL (2998.6–3761.4), and elimination half-life (t½) was 11.3 h (9.8–13.1; Table 3).
3.3 Safety
Brexanolone was generally well tolerated in this population of healthy adult lactating women. There were no SAEs, TEAEs, or deaths, and no clinically significant laboratory findings were reported. Seven (58%) of the 12 participants experienced at least one TEAE; all TEAEs were mild (n = 5) or moderate in severity (n = 2). Two TEAEs (nausea and abnormal dreams; n = 1 each) were considered by the investigator to be related to the study drug. The most frequently reported TEAEs (occurring in > 1 participant) were infusion-site pain (50%), infusion-site swelling (50%), infusion-site erythema (33%), and headache (17%). One participant discontinued the study drug because of TEAEs (edema, pain, and redness at the injection site) but continued in the study and completed all assessments, including collection of breast milk.
3.4 PopPK Modeling
The parameter values for the final model and validation of the model are described in the ESM. The overall PK profile was best described by a two-compartment model with linear elimination and distribution. A partition coefficient describing the gradient in concentrations between milk and plasma was estimated to be 1.36 (milk:plasma). The final model was qualified by goodness-of-fit criteria and plots, and by visual predictive checks for BRX60 and BRX90 (Supplementary Fig. S1, see ESM). The only covariate included in the final model was body weight (Supplementary Fig. S2, see ESM). For a typical patient with a median weight of 82.9 kg, clearance was 89.8 L/h, and the steady-state volume of distribution (Vss) was 587 L. There were no apparent differences in exposure to brexanolone (AUC and Cmax) in patients with or without concomitant antidepressant treatment (Supplementary Fig. S3, see ESM).
3.5 Pharmacokinetics Simulations
Allopregnanolone concentrations in milk and plasma were simulated using median values and the 5th and 95th percentiles from the final PopPK model (Fig. 3a, b). The model characterized milk and plasma data from the 547-CLP-108 study well. Milk and plasma allopregnanolone levels showed a linear relationship and were independent of concentration, time, or expressed milk volume. The model predicted milk allopregnanolone (combined natural and synthetic) levels < 10 ng/mL 36 h after cessation of brexanolone treatment in 95% of the participants. Predicted milk concentrations late in the profile showed apparent under-prediction, likely as the result of samples affected by the contribution of undetected endogenous allopregnanolone in milk at these late time points (Fig. 3a).
A PopPK model of breast milk and plasma allopregnanolone levels. Simulated allopregnanolone concentrations in a breast milk and b plasma using median values and the 5th and 95th percentiles from the final model. Individual points correspond to observed data from Study 547-CLP-108. PopPK population pharmacokinetics
Allopregnanolone concentrations increased during dose up-titration and decreased during dose taper similarly in breast milk and plasma (Fig. 4). Figure 4a, b show the simulated concentration of plasma allopregnanolone vs time in the BRX60 and BRX90 infusion groups, respectively. Figure 4c, d show the simulated concentration of breast milk allopregnanolone versus time in the BRX60 and BRX90 infusion groups, respectively. After infusion with brexanolone, there was a rapid systemic clearance of allopregnanolone and a large steady-state volume of distribution. Interindividual variability in systemic clearance was small, whereas interindividual variability in central volume of distribution was large, likely owing to uncertainty in the estimation of other distribution parameters. Using estimated exposures to allopregnanolone in plasma and breast milk, the median (range) RID calculated for the BRX90 group for hours 24–48 of the infusion period (period with the highest infusion rate) was 0.69% (0.18%–1.3%) (Table 4).
Pharmacokinetics simulation of brexanolone concentrations over time. a Plasma at the 60 µg/kg/h infusion rate, b plasma at the 90 µg/kg/h infusion rate, c breast milk at the 60 µg/kg/h infusion rate, and d breast milk at the 90 µg/kg/h infusion rate. a, b Includes 1000 simulated individual profiles. Body weight was resampled from the observed weight distribution in the study population. Median and 95% prediction intervals of simulated data are illustrated. The final PopPK model in patients with PPD was used to simulate 1000 profiles for the c 60 µg/kg/h and d 90 µg/kg/h regimens. The body weights, which were used for computing the dose, were sampled from the population of participants in the PopPK model. The plots show the median (solid line) and the 95% prediction interval (shaded portion). PopPK population pharmacokinetics, PPD postpartum depression
4 Discussion
This study evaluated allopregnanolone levels in the plasma and breast milk of 12 healthy adult lactating women who received a 60-hour infusion of brexanolone. Plasma concentrations of allopregnanolone exhibited linear PK, and the temporal profile in breast milk paralleled the plasma profile closely, albeit with slightly higher concentrations. Plasma allopregnanolone concentrations were undetectable 3 days after the end of the infusion period. PK [21] and safety [14, 15] parameters of brexanolone in this study were consistent with those in prior studies. A structural PopPK model was constructed using data from three randomized, double-blind, placebo-controlled trials; one open-label study; and the current study to describe the milk and plasma allopregnanolone levels in the study participants, and the results were then extended across the available BRX90 study database of patients with PPD from the start of the infusion through day 7. The PopPK model effectively characterized plasma and breast milk data from participants in this study, with brexanolone concentrations increasing during dose titration and declining during dose taper similarly in plasma and milk. Outputs from the model further demonstrated that allopregnanolone profile in breast milk followed the plasma profile closely. Using parameters from the model, the RID was low (maximum value, 1.3%), suggesting a low risk to breastfed infants.
PPD is associated with multiple negative consequences for mothers and children [8, 33,34,35], and standard-of-care antidepressants are among the most frequently prescribed medications during the postpartum period [26]. Previous reports have shown that patients with severe PPD who received brexanolone infusion showed a significant and rapid reduction in their 17-item Hamilton Rating Scale for Depression total score compared with placebo, with a durable response [14, 15, 21]. A double-blind, placebo-controlled phase II study (202A; NCT02614547) of patients with severe PPD previously showed that of ten patients receiving brexanolone, two who consented to and provided milk samples had allopregnanolone levels below or just above the detection limit by day 7 after the start of brexanolone infusion; on the basis of these data, breastfeeding women participating in a study of intravenous brexanolone for PPD are currently advised to pump and discard breast milk for 7 days after the start of infusion. The current study shows that allopregnanolone levels in breast milk and plasma increased during dose up-titration, decreased during dose taper, and were undetectable 3 days after the end of the infusion period. The PK parameters of brexanolone observed in this healthy volunteer study were consistent with data from an open-label, proof-of-concept study of brexanolone for the treatment of women with severe PPD [21]. Safety data showed similar results from previous phase II and phase III studies in patients with PPD [14, 15, 21] and showed that brexanolone was generally well tolerated in healthy lactating women, with no deaths, SAEs, or clinically significant safety findings.
A PopPK model was constructed to describe the milk and plasma allopregnanolone concentrations in the 12 healthy lactating volunteers in the current study, extending the results across all the available BRX90 clinical study databases. The model successfully described the variability and typical concentration–time profile of allopregnanolone in women treated with brexanolone infusion. The data indicated that the overall plasma concentration–time profile of allopregnanolone is best described by a two-compartment model with linear elimination and distribution. The model predicted that 95% of patients would have milk allopregnanolone levels <10 ng/mL 36 h after treatment cessation. The by-weight model visual predictive checks demonstrated that the estimated parameter covariate relations with body weight were adequate over the weight range, and that the variability in PK parameters was related to individual patient weight. No other significant covariates were identified.
There is robust evidence for the transfer of antidepressants into breast milk, and differences in penetration ratios of antidepressants in breast milk have been associated with oral bioavailability, protein binding, and lipid content [36]. RID—the ratio of infant to maternal dose—is influenced by a number of parameters, including milk intake, feeding intervals, intervals between maternal dosing and feeding, and drug concentrations in milk, all of which contribute to interparticipant and intraparticipant variability [28, 37]. Another important parameter for assessing infant exposure to drugs via breast milk is the absolute infant dose (AID), which is the concentration of drug multiplied by the volume of milk [37]. However, lactating women are typically excluded from drug development processes, and most lactation studies with antidepressants do not report data on AID or milk-to-plasma ratios per FDA guidelines [37]. This results in uncertainty about infant exposure to antidepressants through breast milk [38] as well as poor adherence to pharmacotherapy for PPD. Although the consequences of exposure of the infant to antidepressants through breast milk require careful assessment [27, 37,38,39,40,41,42], the difficulty of conducting large-scale PK studies in a population of breastfeeding mothers has spurred the development of model-based simulation approaches to predict infant exposure to drugs via breast milk [29].
PopPK models have been used previously to study infant exposure via breast milk to the selective serotonin reuptake inhibitor fluoxetine and its metabolite norfluoxetine [28, 29]. Breast milk fluoxetine data were simulated in 1000 mother–infant pairs based on a PopPK model constructed with plasma and breast milk fluoxetine concentrations from 24 women treated with fluoxetine. This approach was shown to be feasible for evaluation of RID for fluoxetine [28]. A PopPK model was also recently constructed to predict the exposure of infants to escitalopram through breast milk [43].
Model parameters were used to calculate RID for brexanolone during the infusion period. An RID < 10% was previously reported as safe based on the number of adverse drug reaction reports in a literature search encompassing data on 205 drugs being an order of magnitude less than the weight-adjusted dose to the mother [32, 44]. AEs were more frequently reported in infants exposed to fluoxetine, which has RID values of approximately 10%, compared with sertraline, which has RID values of 1–3% [39]. These data suggest that the calculated maximum RID of 1.3% for brexanolone reported here represents a low risk to breastfed infants. Additionally, the oral bioavailability of brexanolone is < 5% in adults [45], suggesting a low potential for breastfed infant exposure. Overall, these findings offer support for the labeled dosing recommendations, weight-based dosing, and other PK-related labeling recommendations for brexanolone.
The current study has some limitations. First, the lipid content of breast milk may be an important consideration; concentrations of lipophilic antidepressants vary considerably between foremilk and hindmilk, with their penetration ratios being higher in hindmilk than in foremilk [36]. Second, the current study did not evaluate penetration ratios of brexanolone into breast milk. Third, the current study did not assess milk production prior to brexanolone treatment.
5 Conclusions
Allopregnanolone concentrations in breast milk followed plasma concentrations closely, with a rapid decline after the end of the 60-h infusion to undetectable levels 3 days after the end of infusion. The results from the PopPK model reported here suggest that the model characterized milk and plasma data well. These data suggest that the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone administration or from the mother’s underlying condition. Although no data on the potential effects of brexanolone on breastfed infants currently exist, the risk of adverse events in the infant due to brexanolone is likely to be low, owing to the low RID (maximum of 1.3%) and the low oral bioavailability (< 5%) of brexanolone in adults. Overall PK and safety results were consistent with those of prior studies [14, 15] and are included in the brexanolone labeling [23].
References
Ko JY, Rockhill KM, Tong VT, Morrow B, Farr SL. Trends in postpartum depressive symptoms—27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153–8. https://doi.org/10.15585/mmwr.mm6606a1.
American Psychiatric Institution. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Association; 2013.
Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel SC. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. 2015;11:99–137. https://doi.org/10.1146/annurev-clinpsy-101414-020426.
Bauman BL, Ko JY, Cox S, D’Angelo Mph DV, Warner L, Folger S, et al. Vital signs: postpartum depressive symptoms and provider discussions about perinatal depression—United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(19):575–81. https://doi.org/10.15585/mmwr.mm6919a2.
Posmontier B. Functional status outcomes in mothers with and without postpartum depression. J Midwifery Womens Health. 2008;53(4):310–8. https://doi.org/10.1016/j.jmwh.2008.02.016.
Barkin JL, Wisner KL, Bromberger JT, Beach SR, Wisniewski SR. Factors associated with postpartum maternal functioning in women with positive screens for depression. J Womens Health (Larchmt). 2016;25(7):707–13. https://doi.org/10.1089/jwh.2015.5296.
McLearn KT, Minkovitz CS, Strobino DM, Marks E, Hou W. Maternal depressive symptoms at 2 to 4 months post partum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279–84. https://doi.org/10.1001/archpedi.160.3.279.
Moore Simas TA, Huang MY, Packnett ER, Zimmerman NM, Moynihan M, Eldar-Lissai A. Matched cohort study of healthcare resource utilization and costs in young children of mothers with postpartum depression in the United States. J Med Econ. 2020;23(2):174–83. https://doi.org/10.1080/13696998.2019.1679157.
Balbierz A, Bodnar-Deren S, Wang JJ, Howell EA. Maternal depressive symptoms and parenting practices 3-months postpartum. Matern Child Health J. 2015;19(6):1212–9. https://doi.org/10.1007/s10995-014-1625-6.
Valla L, Wentzel-Larsen T, Smith L, Birkeland MS, Slinning K. Association between maternal postnatal depressive symptoms and infants’ communication skills: a longitudinal study. Infant Behav Dev. 2016;45(Pt A):83–90. https://doi.org/10.1016/j.infbeh.2016.10.001.
Koutra K, Chatzi L, Bagkeris M, Vassilaki M, Bitsios P, Kogevinas M. Antenatal and postnatal maternal mental health as determinants of infant neurodevelopment at 18 months of age in a mother-child cohort (Rhea Study) in Crete, Greece. Soc Psychiatry Psychiatr Epidemiol. 2013;48(8):1335–45. https://doi.org/10.1007/s00127-012-0636-0.
Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26(4):289–95. https://doi.org/10.1016/j.genhosppsych.2004.02.006.
Schweizer-Schubert S, Gordon JL, Eisenlohr-Moul TA, Meltzer-Brody S, Schmalenberger KM, Slopien R, et al. Steroid hormone sensitivity in reproductive mood disorders: on the role of the GABAA receptor complex and stress during hormonal transitions. Front Med (Lausanne). 2020;7: 479646. https://doi.org/10.3389/fmed.2020.479646.
Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058–70. https://doi.org/10.1016/S0140-6736(18)31551-4.
Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480–9. https://doi.org/10.1016/S0140-6736(17)31264-3.
Deligiannidis KM, Kroll-Desrosiers AR, Tan Y, Dubuke ML, Shaffer SA. Longitudinal proneuroactive and neuroactive steroid profiles in medication-free women with, without and at-risk for perinatal depression: a liquid chromatography-tandem mass spectrometry analysis. Psychoneuroendocrinology. 2020;121: 104827. https://doi.org/10.1016/j.psyneuen.2020.104827.
Hellgren C, Akerud H, Skalkidou A, Backstrom T, Sundstrom-Poromaa I. Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology. 2014;69(3):147–53. https://doi.org/10.1159/000358838.
Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59(2):207–13. https://doi.org/10.1016/j.neuron.2008.06.019.
Martinez Botella G, Salituro FG, Harrison BL, Beresis RT, Bai Z, Blanco MJ, et al. Neuroactive Steroids. 2. 3alpha-hydroxy-3beta-methyl-21-(4-cyano-1h-pyrazol-1’-yl)-19-nor-5beta-pregnan-20 -one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (gamma-aminobutyric acid)A receptor. J Med Chem. 2017;60(18):7810–9. https://doi.org/10.1021/acs.jmedchem.7b00846.
Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, et al. Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med. 2019;381(10):903–11. https://doi.org/10.1056/NEJMoa1815981.
Kanes SJ, Colquhoun H, Doherty J, Raines S, Hoffmann E, Rubinow DR, et al. Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Hum Psychopharmacol. 2017. https://doi.org/10.1002/hup.2576.
Rosenthal ES, Claassen J, Wainwright MS, Husain AM, Vaitkevicius H, Raines S, et al. Brexanolone as adjunctive therapy in super-refractory status epilepticus. Ann Neurol. 2017;82(3):342–52. https://doi.org/10.1002/ana.25008.
ZULRESSO (brexanolone) injection for intravenous use [prescribing information]. Cambridge, MA: Sage Therapeutics, Inc. 2019.
Margulis AV, Kang EM, Hammad TA. Patterns of prescription of antidepressants and antipsychotics across and within pregnancies in a population-based UK cohort. Matern Child Health J. 2014;18(7):1742–52. https://doi.org/10.1007/s10995-013-1419-2.
Ververs T, Kaasenbrood H, Visser G, Schobben F, de Jong-vanden Berg L, Egberts T. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol. 2006;62(10):863–70. https://doi.org/10.1007/s00228-006-0177-0.
Schoretsanitis G, Spigset O, Stingl JC, Deligiannidis KM, Paulzen M, Westin AA. The impact of pregnancy on the pharmacokinetics of antidepressants: a systematic critical review and meta-analysis. Expert Opin Drug Metab Toxicol. 2020;16(5):431–40. https://doi.org/10.1080/17425255.2020.1750598.
Schoretsanitis G, Westin AA, Deligiannidis KM, Spigset O, Paulzen M. Excretion of antipsychotics into the amniotic fluid, umbilical cord blood, and breast milk: a systematic critical review and combined analysis. Ther Drug Monit. 2020;42(2):245–54. https://doi.org/10.1097/FTD.0000000000000692.
Panchaud A, Garcia-Bournissen F, Csajka C, Kristensen JH, Taddio A, Ilett KF, et al. Prediction of infant drug exposure through breastfeeding: population PK modeling and simulation of fluoxetine exposure. Clin Pharmacol Ther. 2011;89(6):830–6. https://doi.org/10.1038/clpt.2011.23.
Tanoshima R, Bournissen FG, Tanigawara Y, Kristensen JH, Taddio A, Ilett KF, et al. Population PK modelling and simulation based on fluoxetine and norfluoxetine concentrations in milk: a milk concentration-based prediction model. Br J Clin Pharmacol. 2014;78(4):918–28. https://doi.org/10.1111/bcp.12409.
Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–77. https://doi.org/10.1176/appi.ajp.2011.10111704.
Rowe H, Baker T, Hale TW. Maternal medication, drug use, and breastfeeding. Child Adolesc Psychiatr Clin N Am. 2015;24(1):1–20. https://doi.org/10.1016/j.chc.2014.09.005.
Bennett P, Notarianni LJ. Risk from drugs in breast milk: an analysis by relative dose. Br J Clin Pharmacol. 1996;42:673P-4P (abstract).
Accortt EE, Cheadle ACD, Dunkel SC. Prenatal depression and adverse birth outcomes: an updated systematic review. Matern Child Health J. 2015;19(6):1306–37. https://doi.org/10.1007/s10995-014-1637-2.
Netsi E, Pearson RM, Murray L, Cooper P, Craske MG, Stein A. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiat. 2018;75(3):247. https://doi.org/10.1001/jamapsychiatry.2017.4363.
Epperson CN, Huang M-Y, Cook K, Gupta D, Chawla A, Greenberg PE, et al. Healthcare resource utilization and costs associated with postpartum depression among commercially insured households. Curr Med Res Opin. 2020;36(10):1707–16. https://doi.org/10.1080/03007995.2020.1799772.
Schoretsanitis G, Westin AA, Stingl JC, Deligiannidis KM, Paulzen M, Spigset O. Antidepressant transfer into amniotic fluid, umbilical cord blood and breast milk: a systematic review and combined analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2021;107: 110228. https://doi.org/10.1016/j.pnpbp.2020.110228.
den Besten-Bertholee D, van der Meer DH, Ter Horst PGJ. Quality of lactation studies investigating antidepressants. Breastfeed Med. 2019;14(6):359–65. https://doi.org/10.1089/bfm.2019.0021.
Delaney SR, Malik PRV, Stefan C, Edginton AN, Colantonio DA, Ito S. Predicting escitalopram exposure to breastfeeding infants: integrating analytical and in silico techniques. Clin Pharmacokinet. 2018;57(12):1603–11. https://doi.org/10.1007/s40262-018-0657-2.
Uguz F. Second-generation antipsychotics during the lactation period: a comparative systematic review on infant safety. J Clin Psychopharmacol. 2016;36(3):244–52. https://doi.org/10.1097/JCP.0000000000000491.
Uguz F, Kirkas A, Aksoy ZK, Yunden S. Use of psychotropic medication during lactation in postpartum psychiatric patients: results from an 8-year clinical sample. Breastfeed Med. 2020;15(8):535–7. https://doi.org/10.1089/bfm.2020.0111.
Fischer Fumeaux CJ, Morisod Harari M, Weisskopf E, Eap CB, Epiney M, Vial Y, et al. Risk-benefit balance assessment of SSRI antidepressant use during pregnancy and lactation based on best available evidence - an update. Expert Opin Drug Saf. 2019;18(10):949–63. https://doi.org/10.1080/14740338.2019.1658740.
Paulzen M, Stingl JC, Augustin M, Sassmannshausen H, Franz C, Grunder G, et al. Comprehensive measurements of intrauterine and postnatal exposure to lamotrigine. Clin Pharmacokinet. 2019;58(4):535–43. https://doi.org/10.1007/s40262-018-0713-y.
Weisskopf E, Guidi M, Fischer CJ, Bickle Graz M, Beaufils E, Nguyen KA, et al. A population pharmacokinetic model for escitalopram and its major metabolite in depressive patients during the perinatal period: prediction of infant drug exposure through breast milk. Br J Clin Pharmacol. 2020;86(8):1642–53. https://doi.org/10.1111/bcp.14278.
Begg EJ, Duffull SB, Hackett LP, Ilett KF. Studying drugs in human milk: time to unify the approach. J Hum Lact. 2002;18(4):323–32. https://doi.org/10.1177/089033402237904.
Brexanolone. In: Drugs and Lactation Database(LactMed) [Internet]. Bethesda MD: National Library of Medicine (US); Last updated March 21, 2022.
Acknowledgements
Medical writing and editorial support were provided by Sean Sheffler-Collins, PhD, and Padma Sridhar, PhD, of Symbiotix, LLC, and funded by Sage Therapeutics, Inc. The authors thank Julie Donovan and Koji Takahashi, PhD, for their contributions to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jeffrey Wald, Ethan Hoffmann, Haihong Li, and Helen Colquhoun are employees of Sage Therapeutics, Inc., and hold stock and/or stock options. Kristina Deligiannidis serves as a consultant to Sage Therapeutics, Inc., and reports grants awarded to Zucker Hillside Hospital/Feinstein Institutes for Medical Research during the conduct of the brexanolone injection and zuranolone clinical trials. Anja Henningsson and Eva Hanze are employees at qPharmetra LLC and serve as consultants to Sage Therapeutics, Inc.
Ethics approval
All clinical studies described herein have been approved by the appropriate Ethics Committees of each participating center and have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
All study participants provided written informed consent prior to participation in the study.
Consent for publication
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available because participants in this study did not agree for their data to be shared publicly.
Code availability
Not applicable.
Author contributions
All authors participated in the interpretation of the study results and in the drafting, critical revision, and approval of the final version of the manuscript. Study design, data collection, and data analyses: JW, AH, EHa, EHo, HL, HC, and KMD. Study monitoring: EHo and HC.
Funding
This study was funded by Sage Therapeutics, Inc.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wald, J., Henningsson, A., Hanze, E. et al. Allopregnanolone Concentrations in Breast Milk and Plasma from Healthy Volunteers Receiving Brexanolone Injection, With Population Pharmacokinetic Modeling of Potential Relative Infant Dose. Clin Pharmacokinet 61, 1307–1319 (2022). https://doi.org/10.1007/s40262-022-01155-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01155-w