Abstract
Background and Objective
There is no licensed treatment for refractory chronic cough; off-label therapies have limited efficacy and can produce adverse effects. Excessive adenosine triphosphate signaling via P2X3 receptors is implicated in refractory chronic cough, and selective P2X3 receptor antagonists such as eliapixant (BAY 1817080) are under investigation. The objective of the study was to investigate the safety and tolerability of ascending repeated oral doses of eliapixant in healthy volunteers.
Methods
We conducted a repeated-dose, double-blind, randomized, placebo-controlled study in 47 healthy male individuals. Subjects received repeated twice-daily ascending oral doses of eliapixant (10, 50, 200, and 750 mg) or placebo for 2 weeks. The primary outcome was frequency and severity of adverse events. Other outcomes included pharmacokinetics and evaluation of taste disturbances, which have occurred with the less selective P2X3 receptor antagonist gefapixant.
Results
Peak plasma concentrations of eliapixant were reached 3–4 h after administration of the first and subsequent doses. With multiple dosing, steady-state plasma concentrations were reached after ~ 6 days, and plasma concentrations predicted to achieve ≥ 80% P2X3 receptor occupancy (the level required for efficacy) were reached at 200 and 750 mg. Increases in plasma concentrations with increasing doses were less than dose proportional. After multiple dosing, mean plasma concentrations of eliapixant showed low peak–trough fluctuations and were similar for 200- and 750-mg doses. Eliapixant was well tolerated with a low incidence of taste-related adverse events.
Conclusions
Eliapixant (200 and 750 mg) produced plasma concentrations that cover the predicted therapeutic threshold over 24 h, with good safety and tolerability. These results enabled eliapixant to progress to clinical trials in patients with refractory chronic cough.
Clinical Trial Registration
Clinicaltrials.gov: NCT03310645 (initial registration: 16 October, 2017).
Plain Language Summary
There are few effective treatments for patients with a long-term (chronic) cough. It is thought that chronic cough is caused by nerves becoming oversensitive, wrongly causing a cough when there is no need. We tested a new drug called eliapixant in 47 healthy men. Eliapixant reduces the excessive nerve signaling responsible for chronic cough. We looked for side effects of eliapixant and measured how it behaves in the body. In particular we looked for side effects relating to the sense of taste because gefapixant, a similar drug to eliapixant, can affect taste. Participants took one of four eliapixant doses or a placebo twice daily for 2 weeks. The highest levels of eliapixant in the blood were seen 3–4 h after taking the drug, and stable concentrations were seen after about 6 days. At the two highest doses, eliapixant reached concentrations in the body that should be high enough to work in patients with chronic cough. Side effects were generally similar between eliapixant and placebo. Taste-related side effects were mild and went away without needing treatment. The positive results of this study meant that eliapixant could be tested in patients with chronic cough.
AbstractSection Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Higher doses of eliapixant led to plasma concentrations predicted to achieve at least 80% P2X3 receptor occupancy, the predicted threshold required for efficacy. |
Eliapixant was well tolerated at all doses investigated. Taste-related adverse events were infrequent and mild in severity. |
Based on the results of this study, clinical development of eliapixant was progressed to include patients with refractory chronic cough. |
1 Introduction
Adenosine triphosphate (ATP) signaling occurs via purinergic P2 receptors, designated P2X [1, 2]. P2X receptor subunits (P2X1–P2X7) occur as homotrimers (e.g., P2X3) and heterotrimers (e.g., P2X2/3) [1,2,3]. Excessive ATP signaling via P2X3 receptors has been implicated in many disorders, including refractory chronic cough (RCC), endometriosis, overactive bladder (OAB), and diabetic neuropathic pain [4,5,6,7,8,9,10]. The P2X2/3 heterotrimer is an important mediator of taste sensation [11,12,13].
Refractory chronic cough is defined as cough persisting for 8 weeks or longer despite investigation and treatment according to guidelines [14]. Up to 40% of patients attending respiratory or specialist cough clinics report RCC [15,16,17]. Chronic cough produces numerous physical, psychological, and psychosocial morbidities [14, 18, 19]. There is no licensed treatment for RCC, and off-label therapies have unsatisfactory efficacy and tolerability, highlighting a need for new treatments [14]. The importance of P2X3 receptor pathways in RCC was first highlighted by clinical trials of gefapixant [10, 20,21,22,23]. Gefapixant is an antagonist at the P2X3 homotrimer and the P2X2/3 heterotrimer, with half-maximal inhibitory concentrations (IC50) of 153 nM for P2X3 and 220 nM for P2X2/3 in patch clamp studies [24].
In phase III trials, gefapixant 45 mg twice daily (BID) reduced awake cough frequency by 18% vs placebo at week 12 and by 15% vs placebo at week 24, but was associated with taste-related adverse events (AEs), mainly dysgeusia [23, 25], attributed to P2X2/3 receptor blockade [10, 20,21,22].
A selective P2X3 receptor antagonist could have therapeutic potential in RCC, with less risk of taste disturbances from P2X2/3 receptor blockade. Eliapixant (BAY 1817080) is a potent P2X3 receptor antagonist with in vitro IC50 values of 8–10 nM and 129–163 nM for P2X3 and P2X2/3 receptors, respectively [26], representing approximately 20-fold selectivity for P2X3 over P2X2/3 receptors.
A single-dose, first-in-human (FiH) study of eliapixant in healthy volunteers was performed using an immediate-release tablet formulation (NCT02817100; unpublished data, Bayer AG, Berlin, Germany). The results showed food-dependent and non-dose-linear pharmacokinetics with a time to reach maximum observed drug concentration of 1.5–3 h and a terminal half-life (t½) of 24–59 h. Greater exposure of eliapixant was observed in a fed vs fasting state (4.1-fold increase in maximum observed drug concentration [Cmax] and 2.7-fold increase in area under the plasma concentration–time curve [AUC]). Exposure was less than dose proportional in the fed state (across the 10–800 mg dose range) and the fasted state. Eliapixant had minimal effects on taste perception.
The FiH study was followed by a two-part phase I/IIa study (NCT03310645). Here, we report the phase I study part, which investigated the pharmacokinetics, taste alterations, safety, and pharmacodynamics of multiple doses of eliapixant in healthy subjects. The phase IIa part in patients with RCC demonstrated that eliapixant at doses ≥ 50 mg BID significantly reduced cough frequency and severity and was well tolerated with acceptable rates of taste-related events, showing target engagement and indicating that P2X3 and not P2X2/3 is a relevant receptor for treating RCC [27]. The results also suggested the likely dose range in other potential indications. Eliapixant is undergoing phase II trials in other conditions including endometriosis (NCT04614246) and OAB (NCT04545580; EudraCT 2019-002575-34).
2 Methods
2.1 Study Design and Participants
This repeated-dose, double-blind, randomized, placebo-controlled, dose-escalation study was conducted at a UK clinical pharmacology unit. Healthy male individuals aged 18–45 years with a body mass index of 18–30 kg m–2 were eligible.
2.2 Outcomes
The primary outcome was the frequency and severity of AEs. Other outcomes included pharmacokinetic assessments, taste assessments, and pharmacodynamic evaluation by an exploratory ATP cough challenge test.
2.3 Pharmacokinetic Model Simulations for Selection of Doses
The current study used the same immediate-release tablet formulation as the FiH study. Based on the FiH results, a population pharmacokinetic model for multiple dosing of eliapixant was parameterized (see the Electronic Supplementary Material [ESM]). In silico simulated doses and dosing regimens were selected to achieve trough plasma drug concentrations (Ctrough) producing the following levels of P2X3 receptor occupancy (RO): RO > 20 and < 50% at the typical Ctrough with the lowest dose of eliapixant; RO > 50 and < 80% at the typical Ctrough with the second dose; RO > 80% at the typical Ctrough with the third dose; RO > 80 for ≥ 90% of the population with the highest dose.
Receptor occupancy > 80% is the expected threshold for efficacy based on preclinical studies in which RO of > 80% with P2X3 inhibitors was needed to obtain strong significant effects on complete Freund’s adjuvant-induced hyperalgesia in rats (unpublished data, Bayer AG, Berlin, Germany). Percentage RO was calculated based on the IC50 value of 10 nM for eliapixant for blocking human homomeric P2X3 receptors in an in vitro whole-cell manual patch clamp assay (unpublished data, Bayer AG, Berlin, Germany). To achieve RO > 80% in humans, an unbound minimum target plasma eliapixant concentration of 19 µg L–1 was obtained from the IC50 of 10 nM. Given a mean fraction of eliapixant unbound to human plasma of 13.5%, the calculated total minimum plasma concentration required for RO > 80% is 141 µg L–1 (unpublished data, Bayer AG, Berlin, Germany).
The in silico simulation used the planned dosing regimen for healthy volunteers: each dose was given for 14 days, three times on day 1 and BID from day 2 onwards. Based on the simulations, 10, 50, 200, and 750 mg were selected as loading and maintenance doses to cover the linear range of the concentration–effect relationship including its expected plateau at higher doses. The model-predicted exposure in healthy volunteers is shown in Fig. 1.
Model-based predicted typical exposure for healthy volunteers, compared with the exposure observed with a single dose of 800 mg administered after a high-fat/high-calorie breakfast (black solid line), shown in relation to three levels of inhibitory concentration (IC). Day 0: maintenance dose twice daily (BID), day 1: loading dose three times daily (0, 6, 12 h), days 2–13: maintenance dose BID. Upper left (dose level 1): 10 mg as a loading and maintenance dose. Upper right (dose level 2): 50 mg as a loading and maintenance dose. Lower left (dose level 3): 200 mg as a loading and maintenance dose. Lower right (dose level 4): 750 mg as a loading and maintenance dose. Dashed horizontal lines indicate ICs for 20, 50, and 80% of receptors (IC20, IC50, IC80; the latter is the expected threshold for efficacy)
2.4 Procedures
Subjects were randomized into four cohorts. Each cohort contained up to nine subjects receiving repeated doses of eliapixant (10, 50, 200, and 750 mg) BID and three receiving placebo for 2 weeks. These numbers, giving a total of 48 subjects, were considered sufficient to fulfill the study objectives. For further details of randomization and blinding, see the ESM.
Treatment with each next higher dose level of eliapixant was carried out only after safety, tolerability, and pharmacokinetic assessment of all previous dose levels. Eliapixant or placebo was administered three times on day 1 as a loading dose, BID from day 2 until day 12, and once on day 13. Study treatment was given within 30 min after the start of a meal. Blood samples for pharmacokinetic analyses were taken every 15–240 min for the first 12 h on day 0 and then once on days 1–4, 6, 8, 10, and 11. On day 12, two samples were taken at 12-h intervals with two additional samples at 2-h intervals after the last sample. On day 13, samples were taken every 15–240 min for the first 12 h, followed by once daily on days 14–16, 18, and 20. All samples were stored at − 25 ± 5 °C and analyzed within 33 weeks. Eliapixant in plasma was analyzed using fully validated high-pressure liquid chromatography and tandem mass spectrometry. Concentrations of eliapixant were determined after protein precipitation with acetonitrile/2 mM ammonium acetate containing 0.1% formic acid, including an internal standard ([13C6] eliapixant) followed by liquid chromatography and tandem mass spectrometry. The calibration range was from 1.00 μg L–1 (lower limit of quantification) to 4000 μg L–1 (upper limit of quantification). Quality control samples ranged from 3.00 to 3000 μg L–1 and were determined with an accuracy of 94.2–97.7% and a precision of 4.8–10.2%.
The main pharmacokinetic parameters assessed were the maximum observed drug concentration after multiple dosing and AUC from time 0–12 h with multiple dosing. Additional parameters included AUC from time 0–12 h (AUC(0–12)) and Cmax for the first dose, time to reach maximum observed drug concentration for the first dose and after multiple dosing, t½, AUC from time 0 to the last data point and peak–trough fluctuation for multiple dosing, accumulation ratios calculated from AUC(0–12) after multiple dosing compared with the first dose; and accumulation ratios calculated from Cmax after multiple dosing and Cmax during planned times after the first dose.
Taste assessments were performed using taste strips and a dysgeusia questionnaire. Taste strips (Burghart Messtechnik GmbH, Wedel, Germany) were used pre-dose and before breakfast on days 3 and 13, and assessed sweet, sour, salty, and bitter taste perception using strips with ascending concentrations of sucrose, citric acid, sodium chloride, and quinine hydrochloride, respectively. The strips were placed one at a time in a random order on the anterior third of the subject’s tongue. Subjects were asked to describe the taste by choosing one of five descriptors (sweet, sour, salty, bitter, no taste). The number of correctly identified tastes was summed to produce a taste score. No food intake was allowed before the test. These strips have been validated in the literature [28, 29] and by the sponsor (unpublished data, Bayer AG, Berlin, Germany). The dysgeusia questionnaire was conducted after lunch pre-dose and on days 3 and 13 by asking subjects about the presence of qualitative taste impairments, such as metallic taste or permanent bitter, sour, salty, or sweet taste.
Safety and laboratory assessments included AEs, serious AEs, intensity of AEs, possible relationship to study medication, management of AEs, and outcome of AEs. Adverse events were analyzed using Medical Dictionary for Regulatory Activities preferred terms.
For the pharmacodynamic evaluation, ATP inhalation cough challenge tests were performed pre-dose and on day 13 using nebulized ATP solutions in concentrations of 0.125–512 mg mL–1. Cough frequency was monitored with a VitaloJAK cough recorder. Subjects inhaled doubling concentrations of ATP until the maximum tolerated dose was achieved. Adenosine triphosphate concentrations that induced at least two coughs and at least five coughs within 15 seconds after ATP inhalation were noted.
2.5 Statistical Analysis
Sample size for dose escalation was not based on statistical calculations. As usual for this type of study, sample size was based on experience from previous multiple-dose-escalation studies for other compounds. A sample size of 12 participants at each dose step (eliapixant, n = 9; placebo, n = 3, with placebo patients pooled for statistical evaluation) was considered sufficient to detect safety risks associated with eliapixant and to fulfill the objectives of the study.
Summary statistics are presented per dose step for subjects treated with eliapixant, and for all subjects treated with placebo pooled from the different dose steps. Pharmacokinetic parameters were calculated using WinNonlin version 5.3 on all subjects receiving active therapy. Summary statistics for pharmacokinetic parameters included geometric mean, coefficient of variation, and range. Geometric mean concentration–time curves for all analytes were plotted for each dose and placebo using a semi-logarithmic scale.
Overall taste score and its differences from baseline were analyzed using descriptive statistics by dose group. Point estimates and 95% confidence intervals for the mean difference from baseline were determined for each dose group. Reductions in taste score indicate worsening of subjects’ ability to identify different taste qualities and taste intensities. Further information on study design is available in the ESM.
3 Results
3.1 Participants
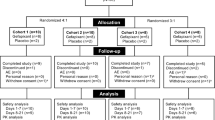
Between 7 December, 2017 and 5 July, 2018, 148 subjects were screened. In total, 101 did not complete screening and were excluded: 99 because of screening failure, one because laboratory results were not available, and one subject withdrew. The most common causes of failure were inability to taste the taste strips (24 subjects) and failure to cough on ATP inhalation (17 subjects). In total, therefore, 47 subjects were randomized: nine each to eliapixant 10, 50, and 200 mg, eight to eliapixant 750 mg, and 12 to placebo (Fig. 2). One subject in the eliapixant 200-mg group discontinued because of an AE (see Safety section) and one subject in the placebo group withdrew for personal reasons. Therefore, 45 subjects completed the study, although all 47 completed a follow-up assessment (Fig. 2) and the trial was completed according to protocol.
Subject disposition. aPremature termination of eliapixant (n =1). Raised liver function tests associated with Epstein–Barr virus infection, not considered related to the study drug. Resolved after drug discontinuation. bPremature termination of placebo (n =1). Withdrawal by subject (personal reasons)
The safety analysis set included all 47 subjects, and the per-protocol set in which taste score was assessed comprised the 45 subjects who completed the study. The ATP cough challenge was performed in a modified per-protocol set (n = 40; one subject dropped out of the study and six had missing or invalid data). The pharmacokinetics set included 34 subjects (all subjects receiving the active drug completed the study, except for one receiving eliapixant 200 mg).
Baseline demographics and characteristics were generally similar between treatment groups (Table 1). Most subjects were white (85%) with a mean (standard deviation) age of 30.6 (7.1) years. Baseline demographics and characteristics of the per-protocol set (Table S1 of the ESM), modified per-protocol set (Table S2 of the ESM), and pharmacokinetics set (Table S3 of the ESM) were comparable with the safety analysis set.
3.2 Pharmacokinetic Analyses
For each of the four dosages, peak plasma concentrations of eliapixant were reached 3–4 h after administration of the first dose (Fig. 3a) and after the last dose. With multiple dosing, steady-state plasma concentrations were reached after approximately 6 days, and plasma concentrations predicted to achieve ≥ 80% P2X3 RO were reached with the 200- and 750-mg doses, as predicted by the pharmacokinetic model (Fig. 3b). Plasma t½ of eliapixant (52–78 h) was similar for all dose regimens (Fig. S1 of the ESM).
Geometric mean (standard deviation) plasma concentrations of eliapixant over a 0–24 h and b day 0–day 20 (semi-logarithmic scale). Upper horizontal line represents the concentration of eliapixant (141 μg L–1) predicted from preclinical/in vitro data to reach 80% P2X3 receptor occupancy, the expected relevant threshold for efficacy (unpublished data, Bayer AG, Berlin, Germany). h hours, LLOQ lower limit of quantification (1 μg L–1), RO80 concentration required to achieve 80% P2X3 receptor occupancy
Pharmacokinetic parameters for the first dose and after multiple dosing are shown in Table 2. Plasma concentrations increased with increasing dose but were less than dose proportional and, after multiple dosing, were comparable for the 200- and 750-mg doses. Accumulation ratios of eliapixant ranged from 4.39 to 4.78 for AUC and from 3.00 to 3.15 for Cmax. Peak–trough fluctuation of plasma eliapixant concentrations after multiple dosing was low, from a mean (coefficient of variation) of 28.1% (44.1%) to 44.1% (21.9%).
3.3 Taste Assessment
Changes from baseline to days 3 and 13 in the overall taste score with eliapixant ranged from + 0.4 to − 1.6, with no clear dose relationship (Fig. 4; Table S4 of the ESM). For the 750-mg dose, this change was nominally significant at day 13 (95% confidence interval − 3.23, − 0.018). With placebo, the changes ranged from − 0.3 to − 0.8. No clinically relevant changes were observed in sensation of the individual taste qualities (sweet, sour, salty, or bitter), with mean changes from baseline for eliapixant from + 0.6 to − 1.1 compared with + 0.3 to − 0.5 for placebo (Table S5 of the ESM). On the taste questionnaire, only one subject (14%) in the eliapixant 200-mg group reported dysgeusia (a continuously sour taste in the mouth) on day 13, which was mild in intensity and resolved by day 29.
3.4 Safety
Adverse events occurred in seven (78%), six (67%), five (56%), six (75%), and nine subjects (75%) with eliapixant 10 mg, 50 mg, 200 mg, 750 mg, and placebo, respectively (Table 3). The majority of AEs were mild in intensity and the most common were headache (30%), medical device site rash (11%), and dysgeusia (9%) in the overall population. None of the dysgeusia events reported was recorded as bothersome to the subject, and the incidence of dysgeusia in the eliapixant groups combined (3/35 subjects; 9%) was similar to that in the placebo group (1/12 subjects; 8%). With eliapixant, the three dysgeusia events were sour taste in two subjects in the 200-mg group, lasting for 2 h and 16 days, respectively, and metallic taste in a subject in the 750-mg group, lasting for 30 min. All taste-related AEs were mild and resolved by the end of the study without treatment.
No severe AEs, serious AEs, or AEs leading to death were reported in the study. One subject was withdrawn from the 200-mg group because of raised liver function tests associated with Epstein–Barr virus infection, not considered related to the study drug, which resolved after drug discontinuation. No other clinically significant changes in laboratory parameters or assessments or vital signs were reported.
3.5 ATP Cough Challenge Test
Treatment with eliapixant (all doses over 2 weeks) had no apparent effects on the results of the ATP cough challenge test. Before treatment, median (range) cough counts at the highest ATP concentration were 15 (5–38), 19 (8–23), 10 (2–44), 8 (0–16), and 13 (0–34) in the eliapixant 10-, 50-, 200-, 750-mg, and placebo arms, respectively. After treatment, median cough counts were 18 (6–36), 14 (4–28), 13 (3–35), 9 (0–23), and 11 (0–28), respectively. Further results for this test are shown in Table S6 of the ESM.
4 Discussion
This study investigated the pharmacokinetics, potential for taste alterations, safety, and pharmacodynamics of ascending repeated doses of eliapixant in healthy male individuals. Peak plasma concentrations of eliapixant were recorded after 3–4 h for all doses. The 200- and 750-mg doses achieved plasma concentrations predicted to achieve ≥ 80% P2X3 RO, which preclinical studies suggested is required for efficacy (unpublished data, Bayer AG, Berlin, Germany). Preclinical data suggest that the concentration required to reach ≥ 80% RO is approximately 20 times higher for P2X2/3 receptors, which are important in mediating taste sensation [11,12,13], than for P2X3 receptors (unpublished data, Bayer AG, Berlin, Germany).
Absence of dose proportionality was shown by AUC(0–12) and Cmax rising less than expected from the increase in dose, with little change between the 200- and 750-mg doses, and by the decreasing dose-normalized AUC(0–12) values over the dosage range, consistent with the single-dose FiH study (unpublished data, Bayer AG, Berlin, Germany). Similar t½ values were observed after multiple doses in the current study as in the FiH study (unpublished data, Bayer AG, Berlin, Germany), which is generally considered to indicate linear pharmacokinetics, although the AUC data suggest non-linear absorption. The pharmacokinetic results were in the expected range of the model predictions and supported use of the same doses (10, 50, 200, and 750 mg BID) for patients with RCC. In the RCC study, plasma concentrations of eliapixant increased between the 200- and 750-mg doses, although not proportionally to the increase in dose [27]. This difference between the RCC study and our results may be explained by the smaller sample size in the current study or by the more restrictive/standardized food conditions at the time of drug administration for the healthy volunteers compared with patients taking tablets at home.
A nominally significant reduction in the overall taste score (− 1.6 points) was observed at 13 days with eliapixant 750 mg, but no adjustment for multiple testing was made, and the difference between this dose and placebo (mean reduction of 0.3 points) is not considered significant. On the taste questionnaire, only one subject reported a continuous taste change. Importantly, all subjects were screened for their ability to taste the strips, and those who could not do so were excluded. Overall, the incidence of dysgeusia was low and similar between eliapixant (3/35 subjects; 9%) and placebo (1/12 subjects; 8%). The taste-related AEs seen with eliapixant were mild, and two of the three events were of short duration (0.5–2 h). The lack of clinically relevant effects of eliapixant on individual taste qualities in the taste strip test is therefore unsurprising. Studies of other P2X3 receptor antagonists have also reported taste-related AEs, although the results cannot be compared directly with ours because of differences in trial designs and populations. The frequency of taste disturbances appears to differ between normal volunteers and patients with chronic cough, possibly because the latter have vagal afferent hypersensitivity [30, 31]. The current study cannot therefore be interpreted as predicting no excess taste effects in clinical studies of eliapixant. A single-dose study of gefapixant 100 mg in 12 healthy subjects reported dysgeusia in nine subjects (75%) and ageusia in six subjects (50%), compared with one subject (8%) each with placebo [20]. The frequency of dysgeusia with gefapixant in phase II trials varied from < 10% at 7.5 mg to 88% at 600 mg [10, 20,21,22]. In phase III trials, the frequency of taste-related AEs was 11–20% at 15 mg BID and 58–69% at 45 mg BID, with dysgeusia in 7–12% and 34–43%, respectively [23]. Taste-related AEs with gefapixant have been attributed to effects on the P2X2/3 heterotrimer [24, 31]. The approximately 20-fold selectivity of eliapixant for P2X3 receptors, and its long terminal t½ leading to a low peak–trough fluctuation, may reduce taste-related AEs by maintaining therapeutic concentrations throughout the dosing period while avoiding excessive peak concentrations that could potentially block P2X2/3 heterotrimers. Two other P2X3 receptor antagonists under development, sivopixant and BLU-5937, have approximately 250-fold and 1000-fold selectivity for P2X3 receptors over P2X2/3 receptors, respectively [32, 33]. In a randomized, double-blind, placebo-controlled, phase I study in 90 healthy subjects, BLU-5937 produced taste AEs in 0–6% of subjects receiving doses of 50–100 mg compared with 25–63% at supra-therapeutic doses (400–1200 mg) [34]. Single ascending doses showed linear pharmacokinetics. The phase II RELIEF study of BLU-5937 failed to achieve the primary endpoint of a reduction in awake cough frequency; a prespecified subgroup analysis, however, demonstrated significant cough suppression in patients with a high baseline cough count [35, 36], and numerical improvements in cough severity and quality of life have been reported [37]. Positive data from the phase IIb SOOTHE study of BLU-5937 have been announced [38] but not yet presented. Sivopixant showed more than dose-proportional pharmacokinetics with multiple dosing in healthy male subjects. Taste disturbances were reported in 6.4% of patients with RCC at the 150-mg dose [39]. The lower rates of taste-related AEs with three P2X3 receptor-selective compounds, eliapixant, BLU-5937, and sivopixant, compared with the unselective gefapixant confirm the involvement of P2X2/3 receptors in taste signaling and suggest a potential clinical advantage for the selective agents. However, the distribution and physiology of P2X2 and P2X3 receptors on gustatory nerves in humans are not completely understood, and as P2X3 receptor antagonists bind to a site between two P2X3 monomers [40], effects on heterotrimers containing two P2X3 subunits and one P2X2 subunit cannot be fully excluded.
Throughout the present study, eliapixant was well tolerated. All AEs were mild or moderate in intensity, no serious AEs or deaths were reported, and no clinically significant changes in laboratory parameters or vital signs were seen. Possible reasons for the absence of a clear effect on the ATP challenge cough test include the small sample size and placebo effects. Moreover, some subjects coughed more than 30 times in the 15-s test period, suggesting that they may have been “trying” to cough. Previous studies have shown that responses to the ATP cough challenge are generally less marked in healthy individuals compared with patients who have chronic cough or respiratory conditions such as asthma [31, 41, 42]. This might be because of a lower expression of P2X3 receptors in the upper airways of healthy individuals. In patients with chronic cough, these receptors can be overexpressed, and increased sensitivity to cough challenges with tussive agents can also be driven by phenotypic changes of sensory airway nerves [43]. In contrast to the absence of effect on cough challenge in the current study, eliapixant produced dose-dependent reductions in cough frequency in patients with RCC, albeit with higher rates of dysgeusia than reported here [27]. Patients with RCC may differ from healthy volunteers in taste perception as well as in cough behavior, and airway sensory nerve density is increased in chronic cough [6], suggesting that P2X3 receptors might be enhanced. Results from the two studies therefore cannot be compared.
Other conditions in which P2X3 receptor antagonism may be useful include endometriosis, OAB, and diabetic neuropathic pain. Endometriosis is a chronic estrogen-dependent inflammatory disease characterized mainly by pain, including dysmenorrhea, non-menstrual pelvic pain, dysuria, dyschezia, and/or dyspareunia, affecting 5–10% of women of reproductive age [44,45,46,47]. Current treatments have limited efficacy in the relief of endometriosis-associated pain, and some have significant AEs that can restrict long-term use [48]. The role of neurogenic inflammation and pain in endometriosis [49], high expression of P2X3 receptors in endometrial lesions [7], and the reversal of hyperalgesia by a P2X3 receptor antagonist in an in vivo endometriosis model [50] (also observed in unpublished data, Bayer AG, Berlin, Germany) suggest that such agents may be effective. P2X3 receptors are also implicated in the control of urinary bladder volume reflexes in OAB, which affects up to 33% of women and 16% of men [4, 51, 52]. Overactive bladder negatively affects patients’ quality of life, mental health, and daily activities, urinary incontinence being the most common symptom [51]. Lifestyle measures and pharmacologic treatments have limited efficacy and the latter can produce AEs, highlighting a need for improved treatments [53]. Diabetic neuropathic pain is a consequence of long-term hyperglycemia, affecting approximately 25% of patients with diabetes mellitus [54]. The current standard of care includes antidepressants, anticonvulsants, and opioids, which are all characterized by severe, at times dose-limiting central AEs and/or the potential for addiction [55, 56]. P2X3 receptor antagonists show efficacy in streptozotocin-induced diabetic neuropathy models, suggesting that they may be effective in treating diabetic neuropathic pain [8, 9].
Limitations of the present study include the small sample size and short duration of treatment and follow-up. The use of male volunteers only, while necessary for this type of study, may have affected the results, as men cough less than women in protussive tests [57] and have lower taste sensitivity than women [58]. Women form the majority of patients with RCC in most populations [59, 60] and in clinical trials of P2X3 receptor antagonists [10, 20,21,22, 27, 39]. The inclusion criteria for the present study specified a cough response to ATP defined as two or more coughs induced by an ATP concentration ≤ 128 mg mL–1. This is not expected to have influenced pharmacokinetics or general safety, but it is unknown whether there is any correlation between cough sensitivity to ATP and sensitivity for taste AEs. If there is such a relationship, it seems likely that it is a direct correlation, which would imply that the subjects in the present study have increased sensitivity for taste-related AEs. The study of gefapixant in healthy volunteers excluded subjects who did not cough more than twice at the two highest concentrations of test solution (ATP 100 mM ≈ 50 mg mL–1 and 300 mM ≈ 150 mg mL–1), as well as patients who did not cough at all during the challenge test [20]. The two studies therefore had similar thresholds for ATP sensitivity. It could be speculated that taste AEs perceived by subjects receiving gefapixant led them to expect suppression of their cough, and therefore they coughed less.
5 Conclusions
This study showed that higher doses (200 and 750 mg) of eliapixant produced plasma concentrations predicted to achieve at least 80% P2X3 RO, the threshold required for efficacy predicted by preclinical data, and were well tolerated with a low incidence of taste-related AEs. The multiple-dose plasma exposure of eliapixant was in accordance with single-dose pharmacokinetics observed in the FiH study with the same tablet formulation (unpublished data, Bayer AG, Berlin, Germany). These results enabled eliapixant to progress to trials in patients with RCC.
References
Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99(1):16–34.
Burnstock G. Purine and purinergic receptors. Brain Neurosci Adv. 2018;6(2):2398212818817494.
North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–80.
Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407(6807):1011–5.
Prado FC, Araldi D, Vieira AS, Oliveira-Fusaro MC, Tambeli CH, Parada CA. Neuronal P2X3 receptor activation is essential to the hyperalgesia induced by prostaglandins and sympathomimetic amines released during inflammation. Neuropharmacology. 2013;67:252–8.
Shapiro CO, Proskocil BJ, Oppegard LJ, Blum ED, Kappel NL, Chang CH, et al. Airway sensory nerve density is increased in chronic cough. Am J Respir Crit Care Med. 2021;203(3):348–55.
Ding S, Zhu L, Tian Y, Zhu T, Huang X, Zhang X. P2X3 receptor involvement in endometriosis pain via ERK signaling pathway. PLoS ONE. 2017;12(9):e0184647.
Migita K, Moriyama T, Koguchi M, Honda K, Katsuragi T, Takano Y, et al. Modulation of P2X receptors in dorsal root ganglion neurons of streptozotocin-induced diabetic neuropathy. Neurosci Lett. 2009;452(2):200–3.
Xu GY, Li G, Liu N, Huang LY. Mechanisms underlying purinergic P2X3 receptor-mediated mechanical allodynia induced in diabetic rats. Mol Pain. 2011;7:60.
Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385(9974):1198–205.
Vandenbeuch A, Larson ED, Anderson CB, Smith SA, Ford AP, Finger TE, et al. Postsynaptic P2X3-containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice. J Physiol. 2015;593(5):1113–25.
Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310(5753):1495–9.
Kinnamon SC, Finger TE. A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci. 2013;7:264.
Morice AH, Millqvist E, Bieksiene K, Birring SS, Dicpinigaitis P, Domingo Ribas C, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55(1):1901136.
Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest. 2005;127(5):1710–3.
Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371(9621):1364–74.
McGarvey L. The difficult-to-treat, therapy-resistant cough: why are current cough treatments not working and what can we do? Pulm Pharmacol Ther. 2013;26(5):528–31.
French CL, Crawford SL, Bova C, Irwin RS. Change in psychological, physiological, and situational factors in adults after treatment of chronic cough. Chest. 2017;152(3):547–62.
French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998;158(15):1657–61.
Morice AH, Kitt MM, Ford AP, Tershakovec AM, Wu WC, Brindle K, et al. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J. 2019;54(1):1900439.
Smith JA, Kitt MM, Butera P, Smith SA, Li Y, Xu ZJ, et al. Gefapixant in two randomised dose-escalation studies in chronic cough. Eur Respir J. 2020;55(3):1901615.
Smith JA, Kitt MM, Morice AH, Birring SS, McGarvey LP, Sher MR, et al. Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med. 2020;8(8):775–85.
McGarvey L, Birring SS, Morice A, Dicpinigaitis P, Pavord I, Schelfhout J, et al. Two phase 3 randomized clinical trials of gefapixant, a P2X3 receptor antagonist, in refractory or unexplained chronic cough (COUGH-1 and COUGH-2). Eur Respir J. 2020;56(64):3800.
Richards D, Gever JR, Ford AP, Fountain SJ. Action of MK-7264 (gefapixant) at human P2X3 and P2X2/3 receptors and in vivo efficacy in models of sensitisation. Br J Pharmacol. 2019;176(13):2279–91.
Muccino DR, Morice AH, Birring SS, Dicpinigaitis PV, Pavord ID, Assaid C, et al. Design and rationale of two phase 3 randomised controlled trials (COUGH-1 and COUGH-2) of gefapixant, a P2X3 receptor antagonist, in refractory or unexplained chronic cough. ERJ Open Res. 2020;6(4):00284–2020.
Davenport AJ, Neagoe I, Bräuer N, Koch M, Rotgeri A, Nagel J, et al. Eliapixant is a selective P2X3 receptor antagonist for the treatment of disorders associated with hypersensitive nerve fibers. Sci Rep. 2021;11(1):19877.
Morice A, Smith JA, McGarvey L, Birring SS, Parker SM, Turner A, et al. Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: a randomised, placebo-controlled, crossover phase 2a study. Eur Respir J. 2021;58(5):2002240.
Landis BN, Welge-Luessen A, Bramerson A, Bende M, Mueller CA, Nordin S, et al. “Taste strips”: a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J Neurol. 2009;256(2):242–8.
Mueller C, Kallert S, Renner B, Stiassny K, Temmel AF, Hummel T, et al. Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips.” Rhinology. 2003;41(1):2–6.
Morice AH, Millqvist E, Belvisi MG, Bieksiene K, Birring SS, Chung KF, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J. 2014;44(5):1132–48.
Morice A. ATP cough challenge. Pulm Pharmacol Ther. 2019;58:101835.
Kai H, Horiguchi T, Kameyma T, Onodera N, Itoh N, Fujii Y, et al. Discovery of clinical candidate Sivopixant (S-600918): lead optimization of dioxotriazine derivatives as selective P2X3 receptor antagonists. Bioorg Med Chem Lett. 2021;52:128384.
Garceau D, Chauret N. BLU-5937: a selective P2X3 antagonist with potent anti-tussive effect and no taste alteration. Pulm Pharmacol Ther. 2019;56:56–62.
Garceau D, Chauret N, Harvey L. BLU-5937: a highly selective P2X3 homotrimeric receptor antagonist exhibits excellent pharmacokinetic and safety profile including improved taste safety profile in healthy subjects. American Cough Conference. 2019. Available from: https://bellushealth.com/wp-content/uploads/2019-06-07-BLU-5937-Presentation-at-American-Cough-Conference.pdf. Accessed 10 Mar 2022.
BELLUS Health. Phase 2 RELIEF trial of BLU-5937 for the treatment of refractory chronic cough. London International Cough Symposium, 2021. Available from: https://bellushealth.com/wp-content/uploads/ICS2021-Bellus-Health-RELIEF-trial-January-22-2021.pdf. Accessed 9 Mar 2022.
Morice JSAH, Birring SS, Parker S, Marsden PA, Holcomb J, Prenner BM, et al. Improvements in cough frequency over 24 hours with BLU-5937, a selective P2X3 antagonist, in patient subgroups defined by baseline awake cough frequencies. A6. A006 Hot takes from clinical trials in lung disease. 2021;A1019. Available from: https://doi.org/10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A1019. Accessed 17 Apr 2022.
Birring SS, Smith JA, Morice AH, Sher M, Hull JH, Goldsobel AB, et al. Improvements in cough severity and cough-related quality of life in a phase 2 trial (RELIEF) with the P2X3 antagonist BLU-5937 in refractory chronic cough (RCC). Eur Respir J. 2021;58(Suppl. 65):OA1199.
BELLUS Health. BELLUS health announces positive topline results from its phase 2b SOOTHE Trial of BLU-5937 for the treatment of refractory chronic cough. 2021. Available from: https://ir.bellushealth.com/node/10526/pdf. Accessed 9 Mar 2022.
Niimi A, Saito J, Kamei T, Shinkai M, Ishihara H, Machida M, et al. Randomised trial of the P2X(3) receptor antagonist sivopixant for refractory chronic cough. Eur Respir J. 2021. https://doi.org/10.1183/13993003.00725-2021
Wang J, Wang Y, Cui WW, Huang Y, Yang Y, Liu Y, et al. Druggable negative allosteric site of P2X3 receptors. Proc Natl Acad Sci USA. 2018;115(19):4939–44.
Fowles HE, Rowland T, Wright C, Morice A. Tussive challenge with ATP and AMP: does it reveal cough hypersensitivity? Eur Respir J. 2017;49(2):1601452.
Basoglu OK, Pelleg A, Kharitonov SA, Barnes PJ. Contrasting effects of ATP and adenosine on capsaicin challenge in asthmatic patients. Pulm Pharmacol Ther. 2017;45:13–8.
Mazzone SB, McGarvey L. Mechanisms and rationale for targeted therapies in refractory and unexplained chronic cough. Clin Pharmacol Ther. 2021;109(3):619–36.
Viganò P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):177–200.
Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315–24.
Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case–control study. Part 1. BJOG. 2008;115(11):1382–91.
Sinaii N, Plumb K, Cotton L, Lambert A, Kennedy S, Zondervan K, et al. Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil Steril. 2008;89(3):538–45.
Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–12.
Laux-Biehlmann A, d’Hooghe T, Zollner TM. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends Pharmacol Sci. 2015;36(5):270–6.
Yuan M, Ding S, Meng T, Lu B, Shao S, Zhang X, et al. Effect of A-317491 delivered by glycolipid-like polymer micelles on endometriosis pain. Int J Nanomed. 2017;12:8171–83.
Johnston KM, Walker DR, Lakzadeh P. Characterizing the health-related quality of life burden of overactive bladder using disease-specific patient-reported outcome measures: a systematic literature review. Adv Ther. 2019;36(3):548–62.
Ford AP, Undem BJ. The therapeutic promise of ATP antagonism at P2X3 receptors in respiratory and urological disorders. Front Cell Neurosci. 2013;7:267.
Corcos J, Przydacz M, Campeau L, Gray G, Hickling D, Honeine C, et al. CUA guideline on adult overactive bladder. Can Urol Assoc J. 2017;11(5):E142–73.
Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, et al. A comprehensive algorithm for management of neuropathic pain. Pain Med. 2019;20(Suppl. 1):S2-12.
Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008;31(7):1448–54.
Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–54.
Hilton EC, Baverel PG, Woodcock A, Van Der Graaf PH, Smith JA. Pharmacodynamic modeling of cough responses to capsaicin inhalation calls into question the utility of the C5 end point. J Allergy Clin Immunol. 2013;132(4):847–55.
Gudziol H, Hummel T. Normative values for the assessment of gustatory function using liquid tastants. Acta Otolaryngol. 2007;127(6):658–61.
Everett CF, Kastelik JA, Thompson RH, Morice AH. Chronic persistent cough in the community: a questionnaire survey. Cough. 2007;3:5.
Morice AH, Jakes AD, Faruqi S, Birring SS, McGarvey L, Canning B, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J. 2014;44(5):1149–55.
Acknowledgements
Dave Singh, Professor of Respiratory Pharmacology at the University of Manchester, was nominated as the principal investigator during the planning phase of the study and provided advice on the design. We thank Dr. Isabel Reinecke and Dagmar Klein for their population pharmacokinetic model predictions of eliapixant multiple-dose plasma exposure. Medical writing services provided by Richard Murphy, PhD, of Adelphi Communications Ltd, Macclesfield, UK were funded by Bayer AG, Berlin, Germany in accordance with Good Publication Practice (GPP3) guidelines. Jaclyn A. Smith is funded by the National Institute for Health Research Manchester Biomedical Research Centre and a Wellcome Investigator Award, and is a National Institute for Health Research Senior Investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Bayer AG. The funder was responsible for the study design, data collection, data analysis, data interpretation, and study report writing. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit this paper for publication.
Conflict of interest
Christian Friedrich, Klaus Francke, Christian Scheerans, Stefan Klein, and Lueder Fels are employees of Bayer AG, Berlin, Germany. Isabella Gashaw was an employee of Bayer AG, Berlin, Germany, when the study was planned, conducted, and analyzed. Alyn Morice reports grants, personal fees, non-financial support, and other from Bayer AG, and grants, personal fees, non-financial support, and other from Bayer US, during the conduct of the study; personal fees and non-financial support from AstraZeneca, personal fees, non-financial support, and other from Bellus Health, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Chiesi Ltd, grants, personal fees, and non-financial support from GlaxoSmithKline, grants, personal fees, non-financial support, and other from Merck Sharp & Dohme Corp., grants from Menlo Therapeutics, grants, personal fees, and other from NeRRe Therapeutics, grants, personal fees, and non-financial support from Phillips Respironics, grants, personal fees, and non-financial support from Respivant Sciences, Inc., and grants, personal fees, non-financial support, and other from Sanofi, outside the submitted work. Jaclyn A. Smith reports grants and personal fees from Bayer AG during the conduct of the study, personal fees from Algernon Pharmaceuticals, AstraZeneca, and Boehringer Ingelheim, grants and personal fees from Axalbion, Bellus Health, GlaxoSmithKline, Menlo Therapeutics, Merck Sharp & Dohme Corp., NeRRe Therapeutics, Nocion Therapeutics, and Shionogi Inc., outside the submitted work. The VitaloJAK algorithm has been licensed by Manchester University Foundation Trust and the University of Manchester to Vitalograph Ltd. and Vitalograph Ireland (Ltd.). Manchester University Foundation Trust receives royalties, which may be shared with the clinical division in which Jaclyn A. Smith works. Thomas Hummel reports grants from aspUraclip, Berlin, Germany, Smell and Taste Laboratory, Geneva, Switzerland, Sony, Stuttgart, Germany, and Takasago, Paris, France, and personal fees from Frequency Therapeutics, Farmington, CT, USA and Baiafoods, Madrid, Spain, outside the submitted work.
Ethics approval
The protocol and all amendments were reviewed and approved by the West London & GTAC Research Ethics Committee of the Health Research Authority (National Health Service, UK: Reference 17/LO/1103) before the start of the study. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Council for Harmonisation guideline on Good Clinical Practice.
Consent to participate
All participants signed an informed consent form before any study-specific tests or procedures were performed.
Consent for publication
Not applicable.
Availability of data and material
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the European Federation of Pharmaceutical Industries and Associations and Pharmaceutical Research and Manufacturers of America principles for responsible clinical trial data sharing, pertaining to scope, time point, and process of data access. Bayer commits to sharing, upon request from qualified scientific and medical researchers, patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the USA and European Union as necessary for doing legitimate research. This commitment applies to data on new medicines and indications that have been approved by the European Union and US regulatory agencies on or after 1 January, 2014. Interested researchers can use http://www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to perform further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded. Requests for data listed as “unpublished data, Bayer AG, Berlin, Germany” should be made to Christian Friedrich, Director Experimental Medicine Clinician, Bayer AG Research & Development, Pharmaceuticals, Building M004, 525, 13353 Berlin, Germany. Email: christian.friedrich@bayer.com.
Code availability
Not applicable.
Author contributions
CF, IG, SK, LF, CS, JAS, and AM participated in the conception, design, or planning of the study. CF, KF, and AM acquired and analyzed the data. JAS and AM were the coordinating investigators during the study. TH developed the taste strips used in the study. CF, AM, and JAS drafted the manuscript. All authors collaborated in the interpretation of study results and review of the manuscript, and approved the final draft for submission.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Friedrich, C., Francke, K., Gashaw, I. et al. Safety, Pharmacodynamics, and Pharmacokinetics of P2X3 Receptor Antagonist Eliapixant (BAY 1817080) in Healthy Subjects: Double-Blind Randomized Study. Clin Pharmacokinet 61, 1143–1156 (2022). https://doi.org/10.1007/s40262-022-01126-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01126-1