Abstract
Background
Endoxifen is the most important active metabolite of tamoxifen. Several retrospective studies have suggested a minimal or threshold endoxifen systemic concentration of 14–16 nM is required for a lower recurrence rate. The aim of this study was to investigate the feasibility of reaching a predefined endoxifen level of ≥ 16 nM (5.97 ng/mL) over time using therapeutic drug monitoring (TDM).
Methods
This prospective open-label intervention study enrolled patients who started treatment with a standard dose of tamoxifen 20 mg once daily for early breast cancer. An outpatient visit was combined with a TDM sample at 3, 4.5, and 6 months after initiation of the tamoxifen treatment. The tamoxifen dose was escalated to a maximum of 40 mg if patients had an endoxifen concentration < 16 nM. The primary endpoint of the study was the percentage of patients with an endoxifen level ≥ 16 nM at 6 months after the start of therapy compared with historical data, in other words, 80% of patients with endoxifen levels ≥ 16 nM with standard therapy.
Results
In total, 145 patients were included. After 6 months, 89% of the patients had endoxifen levels ≥ 16 nM, compared with a literature-based 80% of patients with endoxifen levels ≥ 16 nM at baseline (95% confidence interval 82–94; P = 0.007). In patients with an affected CYP2D6 allele, it was not always feasible to reach the predefined endoxifen level of ≥ 16 nM. No increase in tamoxifen-related adverse events was reported after dose escalation.
Conclusion
This study demonstrated that it is feasible to increase the percentage of patients with endoxifen levels ≥ 16 nM using TDM. TDM is a safe strategy that offers the possibility of nearly halving the number of patients with endoxifen levels < 16 nM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endoxifen is the most important active metabolite of tamoxifen. Several studies have suggested a minimal or threshold endoxifen systemic concentration of 14–16 nM is required for a lower recurrence rate. |
We studied 145 patients who started with tamoxifen treatment in the adjuvant setting. This study demonstrated the feasibility of therapeutic drug monitoring (TDM)-guided tamoxifen dosing. |
TDM offers the possibility of safely halving the number of patients with subtherapeutic endoxifen levels without introducing additional toxicity. Therefore, physicians are encouraged to implement TDM of tamoxifen in clinical practice, pending further prospective evidence of the role of TDM in recurrence-free and overall survival. |
1 Introduction
Tamoxifen significantly reduces the risk of disease recurrence and mortality in patients with hormone receptor-positive breast cancer [1,2,3]. In premenopausal women, tamoxifen monotherapy (preferably with ovarian suppression) is indicated for a period of 5 years, whereas postmenopausal women are often prescribed a sequential treatment of tamoxifen followed by an aromatase inhibitor [4, 5]. Despite adjuvant endocrine treatment, the disease returns within 5 years in 11–23% of patients and within 15 years in approximately 30% [6, 7].
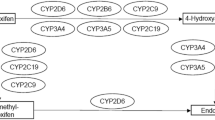
Tamoxifen is a prodrug that is mainly metabolized by cytochrome P450 (CYP)-2D6 and -3A4 into its main active metabolites (Fig. 1). The two main active metabolites (4-hydroxy-tamoxifen and endoxifen) have a 30–100 times greater binding affinity for the estrogen receptor than does tamoxifen. In addition, endoxifen achieves a five to ten times higher plasma concentration than 4-hydroxy-tamoxifen. Therefore, endoxifen is regarded as the most important metabolite [8,9,10,11]. The complex metabolic profile contributes to a high observed interindividual variability in endoxifen concentrations [12].
In several studies, the endoxifen concentration was inversely associated with the risk of breast cancer recurrence. In a retrospective analysis, Madlensky et al. [13] reported a related endoxifen threshold of 16 nM. This study included 1370 pre- and postmenopausal patients with early breast cancer who were treated with tamoxifen in the adjuvant setting for 5 years. Patients with an endoxifen concentration below the lowest quintile (< 16 nM) showed a 26% lower disease-free survival rate than patients with endoxifen levels > 16 nM [13]. In a smaller study among 306 premenopausal patients, the endoxifen levels were divided into quartiles. Compared with endoxifen concentrations in the highest quartile (i.e., > 35 nM), endoxifen concentrations in the lowest quartile (< 14 nM) were associated with an almost two times higher risk of distant recurrence [14]. At the current standard dose of tamoxifen 20 mg once daily (QD), approximately 20–24% of patients have an endoxifen level < 16 nM [13, 15].
Given this exposure–response relationship, dose optimization may reduce the risk of a recurrence of breast cancer. Dose optimization based on CYP2D6 genotype has been attempted since approximately 39% of the variability in endoxifen concentration can be explained by CYP2D6 genotype [16]. However, conflicting results have been reported regarding the association between CYP2D6 genotyping and recurrence rate [17,18,19,20,21,22]. These conflicting results underline the importance of considering other factors that may influence the variability in endoxifen concentration, including concomitant medication, dietary or food supplements, adherence, age, body mass index (BMI), hormonal status, and circadian rhythm [23,24,25,26,27]. Therefore, therapeutic drug monitoring (TDM)-guided dose individualization appears to be a valid method to optimize the endoxifen level [28]. TDM is a tool commonly used to select the right dose of a drug for individuals based on plasma concentrations of the drug or active metabolite [29]. The aim of this prospective study was to investigate the feasibility of increasing the proportion of patients reaching a prespecified endoxifen threshold concentration using TDM. Although the exact threshold of the endoxifen level is currently unknown, we opted for the highest threshold value as described in the literature (≥ 16 nM) to minimize the risk of underdosing [14].
2 Patients and Methods
2.1 Study Design and Population
The TOTAM (TDM Of TAMoxifen) trial was an open-label single-arm intervention study performed at the Erasmus University Medical Center in Rotterdam, the Netherlands, approved by the institutional review board, and registered in the Netherlands Trial Registry (http://www.trialregister.nl; NL6918). Patients who started treatment with a standard dose of tamoxifen 20 mg QD for early breast cancer and who were able and willing to give written informed consent were eligible for participation in this trial. Exclusion criteria were patients receiving tamoxifen for a period longer than 3 months, a starting dose higher than 20 mg QD, a prior diagnosis of endometrial cancer (≤ 3 years prior), or a diagnosis of advanced or metastatic breast cancer.
A hospital visit was combined with TDM samples at 3, 4.5, and 6 months after initiation of the tamoxifen treatment. A hospital visit included registration of comedication or supplements, registration of adverse events using the US National Cancer Institute’s Common Terminology Criteria for Adverse Events version 5 (CTCAEv5), and monitoring of drug adherence using the Morisky Medication Adherence Scale, a widely used self-report questionnaire resulting in a high (score 8), medium (score 6–7), or low (score < 5) adherence rate [30, 31]. Endoxifen trough concentrations at steady-state were sampled and processed to plasma. For quantification of tamoxifen and endoxifen, a validated liquid chromatography tandem mass spectrometry method was used [32]. After laboratory analysis, both tamoxifen and endoxifen concentrations were evaluated by a pharmacist or clinical pharmacologist. Escalation of tamoxifen dose was advised in patients with a high adherence score and a measured endoxifen level < 16 nM. Before increasing the tamoxifen dose, the investigator discussed possible individual factors leading to an endoxifen level below the threshold. Only in patients with nonadherence or potential drug–drug interactions that could be avoided was the tamoxifen dose not initially escalated. In other cases, the tamoxifen dose was escalated to a maximal daily dose of 40 mg as described in the tamoxifen drug label. Patients with an endoxifen level in the range of 12 to < 16 nM were advised to escalate to 30 mg, whereas patients with endoxifen levels < 12 nM were advised to escalate to the maximal daily dose of 40 mg. CYP2D6 genotyping was performed using the Infiniti test (Autogenomics; Carlsbad, CA, USA) and the Quantstudio test (ThermoFisher Scientific; Waltham, MA, USA). Variation in the CYP2D6 gene is responsible for alterations in enzyme activity compared with wild type [33]. CYP2D6 phenotype was assayed in the laboratory on the genetic variants *2–10, *12, *14, *17, *29, and *41; thereafter, patients were classified into four phenotypes based on enzyme function, including ultrarapid metabolizer (UM), extensive metabolizer (EM), intermediate metabolizer (IM), and poor metabolizer (PM). Classification and interpretation were based on the Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and tamoxifen therapy [33].
2.2 Primary Endpoint and Statistical Analysis
The primary endpoint was the percentage of patients with an endoxifen level ≥ 16 nM at 6 months after initiation of adjuvant tamoxifen treatment. The secondary endpoint was the incidence of tamoxifen-related adverse events (CTCAEv5) after dose escalation. This study was powered to demonstrate that 90% of the patients would have endoxifen levels ≥ 16 nM at 6 months after start of tamoxifen by means of TDM-guided dose individualization. For the sample size calculation, we assumed that 80% of the patients had endoxifen levels ≥ 16 nM without TDM-guided dose individualization, as retrieved from literature data [13, 15]. To test this hypothesis with a two-sided α of 0.05, a power of 80%, and applying a continuity correction, at least 118 evaluable patients were required. Patients were defined as evaluable if TDM samples were taken at 3, 4.5, and 6 months after start with tamoxifen treatment. Secondary, the influence of the covariate age categorized as ≤ 45 or ≥ 55 years at the start of tamoxifen treatment on endoxifen exposure was tested using a t-test. The association between CYP2D6 phenotype and BMI with baseline levels of endoxifen were tested by means of analysis of variance. Statistical analysis was performed using SPSS statistics software (version 26, IBM; Armonk, NY, USA).
3 Results
Between January 2018 and June 2019, a total of 145 women with early breast cancer who were treated with adjuvant tamoxifen were enrolled in this trial. To compensate for withdrawals in the period to reach the primary endpoint, more than the required 118 patients were included for the primary endpoint analysis. A total of 136 (94%) participants were evaluable for the primary endpoint analysis as 9 of the 145 patients were switched to an aromatase inhibitor in the meantime (five because of subtherapeutic endoxifen concentrations and four because of tamoxifen-related toxicity). The median age of the patients was 57 years (range 46–66). Prior to tamoxifen treatment, most patients underwent surgery in combination with either radiotherapy, (neo)-adjuvant chemotherapy, or a combination thereof. CYP2D6 was successfully genotyped in all patients and conformed to Hardy–Weinberg equilibrium distribution (P < 0.05). Table 1 provides an overview of the relevant baseline characteristics.
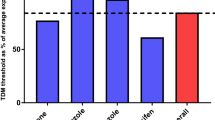
The primary objective of the study was reached by means of TDM-guided dose individualization. After 6 months, 121 of 136 (89%) patients had an endoxifen concentration above the threshold of 16 nM (95% confidence interval [CI] 82–94; P = 0.007). In total, 130 of 145 (90%) participants reached the target concentration or successfully switched to an aromatase inhibitor within the study period of 6 months (Fig. 2). The pharmacokinetic profile of subtherapeutic endoxifen levels over time is depicted in Fig. 3A, and the endoxifen profile of all patients stratified by CYP2D6 phenotype is presented in Fig. 3B.
(A) Pharmacokinetic profile of endoxifen levels below threshold, 3 months (n = 30), 4.5 months (n = 18) and 6 months (n = 15) after start with tamoxifen treatment. (B) Pharmacokinetic profile of endoxifen levels stratified based on CYP2D6 phenotype, 3 months (n = 145), 4.5 months (n = 141), and 6 months (n = 136) after start with tamoxifen treatment. The horizontal line represents the mean endoxifen concentration and the horizontal dashed line represents the predefined endoxifen threshold (≥ 16 nM). EM extensive metabolizer, IM intermediate metabolizer, PM poor metabolizer, T1 3 months after start with tamoxifen, T2 4.5 months after start with tamoxifen, T3 6 months after start with tamoxifen
At the first TDM visit (T1) after 3 months of tamoxifen treatment, 30 (21%) patients had an endoxifen level below the threshold of 16 nM. The tamoxifen dose was escalated to 30 or 40 mg in 27 patients, and three patients received no dose escalation at the discretion of the physician. The mean ± standard deviation concentration of tamoxifen and endoxifen at T1 was 315 ± 99 and 27.7 ± 14.8 nM, respectively (Table 2). At the second TDM visit (T2) after 4.5 months of tamoxifen treatment, 18 patients (tamoxifen dose 20 mg [n = 6], 30 mg [n = 3], and 40 mg [n = 9]) had an endoxifen level < 16 nM. In seven of these patients, the tamoxifen dose was escalated after T2. At T2, the mean endoxifen level of the five patients who had an endoxifen level ≥ 16 nM at T1 and < 16 nM at T2 was 14.4 nM (range 13.8–15.4). Finally, 6 months after initiation of tamoxifen therapy (T3), 15 of 136 (11%) patients (tamoxifen dose 20 mg [n = 8] and 40 mg [n = 7]) had an endoxifen level < 16 nM.
Within the first 6 months of tamoxifen treatment, 31 patients were escalated to a dosage of tamoxifen 30 or 40 mg with one or two dose-escalation steps. After dose escalation, 21 (68%) of these patients reached the threshold. Of the ten patients with subtherapeutic endoxifen concentrations, five (50%) switched to an aromatase inhibitor within 6 months, three (30%) switched to an aromatase inhibitor after 6 months, and two (20%) continued tamoxifen treatment with 40 mg QD. Four participants (mean endoxifen level 23 ± 4.9 nM) switched to an aromatase inhibitor because of tamoxifen-related adverse events while receiving tamoxifen 20 mg QD (Table 3). In our study population, age and BMI had no clear effect on tamoxifen metabolism (P = 0.27 and P > 0.60, respectively). CYP2D6 phenotype (PM, IM, and EM) had a statistically significant effect on endoxifen exposure (P <0.001), with PMs having the lowest systemic endoxifen concentrations (Table 4).
Low endoxifen levels were found in the majority of patients with an IM or PM CYP2D6 phenotype. At T1, 7% of the patients with an EM phenotype had an endoxifen level < 16 nM, whereas 34% of the IMs and 100% of the PMs were below this target. Also, 8% of the participants (with a mean endoxifen concentration of 19.1 nM [range 16.6–19.9]) declined below the threshold during the relatively short study period, despite having a preliminary measurement > 16 nM. After dose escalation, 100% of the EMs, 79% of the IMs, and 36% of the PMs reached the pharmacokinetic target of ≥ 16 nM. The endoxifen concentrations also increased linearly from 10.5 ± 3.3 to 21.8 ± 10.4 nM after dose escalation to tamoxifen 40 mg QD. In the nonescalated group (n = 106), stable endoxifen concentrations over time were observed compared with T1, with mean ± SD endoxifen levels of 32.3 ± 12.5 and 31.9 ± 13.6 nM at baseline and 6 months after initiation of treatment, respectively (Table 2). In the nonescalated group, a relatively low intra-individual variability of 19% was found.
The most commonly reported tamoxifen-related adverse events were hot flashes (61%), arthralgia (19%), fatigue (11%), vaginal dryness (8%), and mood swings (6%) (all CTCAE grade 1) during the first 6 months of this clinical trial. Low-grade toxicity adverse events were often persistent and perceived as limiting. Four patients experiencing these events discontinued their tamoxifen treatment (between T1 and T3). After dose escalation, no increase in tamoxifen-related adverse events, severe or serious adverse events, or treatment discontinuation were reported. Morisky adherence scores in our population were 91%, 5%, and 4% for high, medium, and low adherence, respectively; these results imply a high adherence rate in our study population (Table 1). The use of concomitant CYP2D6 or CYP3A4 inhibitors was limited in the study population. Only two patients used a moderately potent CYP2D6 inhibitor (i.e., sertraline 50 mg QD and quinidine 200 mg twice daily), and one patient used a moderately potent CYP3A4 inhibitor (i.e., diltiazem 120 mg three times daily). One patient used a weak CYP2D6 inhibitor (i.e., citalopram), and 18 patients used a weak CYP3A4 inhibitor (i.e., esomeprazole, omeprazole, or pantoprazole).
4 Discussion
The TOTAM study demonstrated that TDM-guided dose individualization led to a statistically significant and clinically relevant increase in the number of patients with endoxifen levels above the predefined threshold of 16 nM after 6 months of treatment. By using TDM-guided dose individualization for 3 months, nearly 50% of patients with an initial endoxifen level below the predefined threshold reached endoxifen levels ≥16 nM. Therefore, our study offers tools for applying TDM of tamoxifen in clinical practice.
Dose escalation resulted in a significant increase of both tamoxifen and endoxifen concentrations (independent of CYP2D6 status), which is consistent with previous data [12, 34,35,36,37,38]. An Australian dose-escalation study had a higher percentage of patients achieving the threshold than in our data: 94 versus 76% at baseline [15]. However, it should be noted that the defined threshold differed (> 15 vs. ≥ 16 nM), the dose of tamoxifen was increased to a maximum of 60 mg (instead of 40 mg), and the tamoxifen dose was increased in patients with endoxifen levels < 30 nM.
Stratification based on the CYP2D6 genotype showed differences in mean endoxifen levels both before and after dose escalation. After dose escalation, the predefined endoxifen target was achieved in 100% of EMs and 79% of IMs but only 36% of PMs. This result implies that, to achieve therapeutic endoxifen concentrations early in treatment, it is advisable to anticipate—if available—the CYP2D6 genotype status. Our results indicate that PMs might benefit from a starting dose of tamoxifen 40 mg QD combined with TDM and that IMs might benefit from the standard dose combined with TDM. For most extensive metabolizers, tamoxifen 20 mg QD might be sufficient without TDM. An ongoing proof-of-concept study in CYP2D6 IMs and PMs is combining tamoxifen with probenecid (a uridine glucuronyl transferase inhibitor), aiming to reduce the conversion of endoxifen in inactive metabolites (http://www.trialregister.nl, study number NL8444). Another ongoing trial is evaluating the effect of supplementation of Z-endoxifen according to CYP2D6 genotype or plasma levels to reach a predefined endoxifen threshold (NCT03931928). However, a switch to an aromatase inhibitor might also be a valid option for the subgroup of patients with persistently low endoxifen levels after a tamoxifen dose adjustment to 40 mg.
Also in patients with adequate CYP2D6 function, at least one TDM sample is advisable for every patient treated with tamoxifen [16, 39]. Multiple factors other than CYP2D6 genotype can contribute to lower endoxifen levels, including concomitant CYP2D6 inhibitors, adherence, menopausal status, and decreased absorption [23, 40]. However, the low incidence of concomitant use of CYP2D6 inhibitors and the median age of 57 years in our analysis meant that only the CYP2D6 phenotype was highly predictive for endoxifen exposure at baseline. Despite the relatively low intra-individual variability of 19% (as found in our study), 8% of the participants fell below the threshold somewhere during the relatively short study period, even though TDM sample 1 or 2 was > 16 nM (mean endoxifen concentration 19.1 nM [range 16.6–19.9]). This suggests that patients with endoxifen levels in the range of 16–20 nM should be monitored more frequently than patients with endoxifen levels > 20 nM at the first measurement at 3 months after the start of treatment. Long-term (2 years of follow-up) intra-individual data collection in the TOTAM study for tamoxifen pharmacokinetics is ongoing. These data will ultimately represent the pharmacokinetic profile during the first 24 months of treatment with tamoxifen in this cohort of patients.
No increase in the degree or severity of toxicity was observed after the tamoxifen dose was increased. Although the absolute number of patients with increased doses was relatively low, the toxicity data are in agreement with that in the literature. Prospective studies have shown no correlation between tamoxifen dose and incidence of side effects [34, 35, 41, 42]. A longer follow-up should reveal whether this also applies to rare or long-term side effects, such as the risk of developing endometrial carcinoma, deep vein thrombosis, and pulmonary embolism [43,44,45]. Given the likelihood of these serious side effects, a conscious decision was made to increase the dose up to the maximal registered dose of tamoxifen 40 mg QD.
In our study, the degree of adherence was high: 91% of the population received the highest Morisky medication adherence score. Both the adherence questionnaire and tamoxifen levels > 100 nM indicated that participants in this study were highly motivated to adhere to tamoxifen treatment. Another contributor to this high degree of tamoxifen adherence could be the serial TDM, including active counseling by a pharmacist or medical oncologist. However, in real life, the degree of adherence fluctuates between 41 and 88%, so the relevance of TDM can be greater outside the context of a clinical trial or a longer follow-up period [46, 47]. In the literature, discontinuation of tamoxifen therapy is mostly observed during the first year of treatment [46]. For example, a recent study demonstrated that TDM was a useful tool for detecting nonadherence (tamoxifen level < 100 nM) in an early stage of treatment [46,47,48]. As a result of earlier research, almost all concomitant moderate and strong CYP2D6 inhibitors are included in the medication monitoring system of Dutch pharmacies as a monitoring signal [49]. Therefore, minimal use of concomitant CYP2D6 or CYP3A4 inhibitors was noticed in our population.
A methodological strength of our study is the design, with repeated measures of tamoxifen TDM samples compared with mostly single sampling as described in the literature [35]. However, the intensive monitoring could also, paradoxically, be considered a potential limitation. The intensive monitoring strategy potentially positively contributed to the adherence and motivation of patients for tamoxifen treatment. Another strength of our trial was the inclusion of a real-life population of tamoxifen users as well as (1) both pre- and postmenopausal patients, (2) evaluation of an approved on-label dosage (maximum tamoxifen 40 mg QD), and (3) evaluation of a switch to an aromatase inhibitor.
The proportion of patients with breast cancer with subtherapeutic endoxifen levels is determined based on the threshold of 16 nM as first reported by Madlensky et al. [13]. If the true value of this threshold is lower, this could imply that a lower proportion of treatment is categorized as subtherapeutic before TDM. Based on this, the uncertainty regarding the effectiveness of tamoxifen below the current threshold of 16 nM is an important limitation underlying the presented outcome.
Conflicting results have been reported for an endoxifen exposure–response relationship for adjuvant tamoxifen treatment. A preclinical study in mice showed an association between endoxifen levels and tumor growth, and a xenograft model found a dose-dependent association between concentration and degree of gene expression in an MCF7 cell line [50, 51]. These preclinical data support the findings of the retrospective analyses by Madlensky et al. [13] and Saladores et al. [14] with an endoxifen threshold in the range of 14–16 nM. In contrast to these findings, the CYPTAM trial, in which 667 women were treated with tamoxifen, found no association between CYP2D6 genotype and endoxifen concentration in relapse-free survival [52]. However, the design of the study had some limitations, and power was insufficient to conclude that there was no exposure–response relationship. Therefore, an exposure–response relationship remains disputed [53,54,55,56,57]. A prospective randomized controlled TDM study could provide clarity. However, such a trial is probably impossible, since it would require many thousands of patients to participate and a follow-up period of more than a decade [54]. To break out of this potential dead end, physicians are encouraged to implement TDM in clinical practice in the meantime and pending further prospective data. In our opinion, TDM of endoxifen is the most suitable approach for tamoxifen precision dosing because several factors could affect endoxifen concentrations. TDM is mainly recommended in patients with polypharmacy (many concomitant drugs or food supplements), premenopausal patients, and patients diagnosed with an affected CYP2D6 allele. Importantly, an infrastructure to quantify endoxifen concentrations in human plasma is easy to implement in clinical practice.
5 Conclusion
The TOTAM study clearly demonstrated the feasibility of TDM in personalizing tamoxifen treatment in patients with breast cancer. This strategy offers the possibility of safely halving the number of patients with endoxifen levels < 16 nM without introducing additional toxicity.
References
Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9354):296–300.
Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351(9114):1451–67.
Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339(22):1609–18.
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37(5):423–38.
Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, DeCensi A. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37(33):3152–65.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717.
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–84.
Johnson MD, Zuo H, Lee K-H, Trebley JP, Rae JM, Weatherman RV. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85(2):151–9.
Sanchez-Spitman AB, Swen JJ, Dezentje VO, Moes DJ a. R, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics and pharmacogenetics of tamoxifen and endoxifen. Expert Rev Clin Pharmacol. 2019;12(6):523–36.
Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80(1):61–74.
Wang T, Zhou Y, Cao G. Pharmacogenetics of tamoxifen therapy in Asian populations: from genetic polymorphism to clinical outcomes. Eur J Clin Pharmacol. 2021. https://doi.org/10.1007/s00228-021-03088-y.
Jager NGL, Rosing H, Schellens JHM, Linn SC, Beijnen JH. Tamoxifen dose and serum concentrations of tamoxifen and six of its metabolites in routine clinical outpatient care. Breast Cancer Res Treat. 2014;143(3):477–83.
Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89(5):718–25.
Saladores P, Mürdter T, Eccles D, Chowbay B, Zgheib NK, Winter S. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenom J. 2015;15(1):84–94.
Fox P, Balleine RL, Lee C, Gao B, Balakrishnar B, Menzies AM. Dose escalation of tamoxifen in patients with low endoxifen level: evidence for therapeutic drug monitoring—the TADE study. Clin Cancer Res. 2016;22(13):3164–71.
Mürdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89(5):708–17.
Hwang GS, Bhat R, Crutchley RD, Trivedi MV. Impact of CYP2D6 polymorphisms on endoxifen concentrations and breast cancer outcomes. Pharmacogenom J. 2018;18(2):201–8.
Goetz MP, Suman VJ, Hoskin TL, Gnant M, Filipits M, Safgren SL. CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8. Clin Cancer Res. 2013;19(2):500–7.
Kiyotani K, Mushiroda T, Imamura CK, Hosono N, Tsunoda T, Kubo M. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol. 2010;28(8):1287–93.
Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W, Kammler R. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1–98 trial. J Natl Cancer Inst. 2012;104(6):441–51.
Rae JM, Drury S, Hayes DF, Stearns V, Thibert JN, Haynes BP. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104(6):452–60.
Mulder TAM, de With M, Del Re M, Danesi R, Mathijssen RHJ, van Schaik RHN. Clinical CYP2D6 genotyping to personalize adjuvant tamoxifen treatment in ER-positive breast cancer patients: current status of a controversy. Cancers 2021;13:771. https://doi.org/10.3390/cancers13040771.
Binkhorst L, Mathijssen RHJ, Jager A, van Gelder T. Individualization of tamoxifen therapy: much more than just CYP2D6 genotyping. Cancer Treat Rev. 2015;41(3):289–99.
Klopp-Schulze L, Joerger M, Wicha SG, Ter Heine R, Csajka C, Parra-Guillen ZP. Exploiting pharmacokinetic models of tamoxifen and endoxifen to identify factors causing subtherapeutic concentrations in breast cancer patients. Clin Pharmacokinet. 2018;57(2):229–42.
Hussaarts KGAM, Hurkmans DP, Oomen-de Hoop E, van Harten LJ, Berghuis S, van Alphen RJ. Impact of curcumin (with or without piperine) on the pharmacokinetics of tamoxifen. Cancers. 2019;11(3):403.
Mueller-Schoell A, Klopp-Schulze L, Schroth W, Mürdter T, Michelet R, Brauch H. Obesity alters endoxifen plasma levels in young breast cancer patients: a pharmacometric simulation approach. Clin Pharmacol Ther. 2020;108(3):661–70.
Klopp-Schulze L, Mueller-Schoell A, Neven P, Koolen SLW, Mathijssen RHJ, Joerger M. Integrated data analysis of six clinical studies points toward model-informed precision dosing of tamoxifen. Front Pharmacol. 2020;11:283.
de Vries Schultink AHM, Huitema ADR, Beijnen JH. Therapeutic drug monitoring of endoxifen as an alternative for CYP2D6 genotyping in individualizing tamoxifen therapy. Breast. 2018;42:38–40.
Mueller-Schoell A, Groenland SL, Scherf-Clavel O, van Dyk M, Huisinga W, Michelet R. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur J Clin Pharmacol. 2021;77(4):441–64.
Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348–54.
C National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Accessed 14 Jan 2021.
Binkhorst L, Mathijssen RHJ, Ghobadi Moghaddam-Helmantel IM, de Bruijn P, van Gelder T, Wiemer EAC. Quantification of tamoxifen and three of its phase-I metabolites in human plasma by liquid chromatography/triple-quadrupole mass spectrometry. J Pharm Biomed Anal. 2011;56(5):1016–23.
Goetz MP, Sangkuhl K, Guchelaar H-J, Schwab M, Province M, Whirl‐Carrillo M. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin Pharmacol Ther. 103(5):770–7.
Bratherton DG, Brown CH, Buchanan R, Hall V, Kingsley Pillers EM, Wheeler TK. A comparison of two doses of tamoxifen (Nolvadex) in postmenopausal women with advanced breast cancer: 10 mg bd versus 20 mg bd. Br J Cancer. 1984;50(2):199–205.
Hertz DL, Deal A, Ibrahim JG, Walko CM, Weck KE, Anderson S. Tamoxifen dose escalation in patients with diminished CYP2D6 activity normalizes endoxifen concentrations without increasing toxicity. Oncologist. 2016;21(7):795–803.
Barginear MF, Jaremko M, Peter I, Yu C, Kasai Y, Kemeny M. Increasing tamoxifen dose in breast cancer patients based on CYP2D6 genotypes and endoxifen levels: effect on active metabolite isomers and the antiestrogenic activity score. Clin Pharmacol Ther. 2011;90(4):605–11.
Kiyotani K, Mushiroda T, Imamura CK, Tanigawara Y, Hosono N, Kubo M. Dose-adjustment study of tamoxifen based on CYP2D6 genotypes in Japanese breast cancer patients. Breast Cancer Res Treat. 2012;131(1):137–45.
Tamura K, Imamura CK, Takano T, Saji S, Yamanaka T, Yonemori K. CYP2D6 genotype-guided tamoxifen dosing in hormone receptor-positive metastatic breast cancer (TARGET-1): a randomized, open-label, phase II study. J Clin Oncol. 2020;38(6):558–66.
Koolen SLW, Bins S, Mathijssen RHJ. Individualized tamoxifen dose escalation-letter. Clin Cancer Res. 2016;22(24):6300.
Ximenez JPB, de Andrade JM, Marques MP, Coelho EB, Suarez-Kurtz G, Lanchote VL. Hormonal status affects plasma exposure of tamoxifen and its main metabolites in tamoxifen-treated breast cancer patients. BMC Pharmacol Toxicol. 2019;20(Suppl 1):81.
Dezentjé VO, Opdam FL, Gelderblom H, den Hartigh J, Van der Straaten T, Vree R. CYP2D6 genotype- and endoxifen-guided tamoxifen dose escalation increases endoxifen serum concentrations without increasing side effects. Breast Cancer Res Treat. 2015;153(3):583–90.
Jager NGL, Koornstra RHT, Vincent AD, van Schaik RHN, Huitema ADR, Korse TM. Hot flashes are not predictive for serum concentrations of tamoxifen and its metabolites. BMC Cancer. 2013;13:612.
Perez EA. Safety profiles of tamoxifen and the aromatase inhibitors in adjuvant therapy of hormone-responsive early breast cancer. Ann Oncol. 2007;18(Suppl 8):viii26-35.
Ellis AJ, Hendrick VM, Williams R, Komm BS. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921–34.
Duggan C, Marriott K, Edwards R, Cuzick J. Inherited and acquired risk factors for venous thromboembolic disease among women taking tamoxifen to prevent breast cancer. J Clin Oncol. 2003;21(19):3588–93.
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–78.
Pagani O, Gelber S, Colleoni M, Price KN, Simoncini E. Impact of SERM adherence on treatment effect: International Breast Cancer Study Group Trials 13–93 and 14–93. Breast Cancer Res Treat. 2013;142(2):455–9.
Pistilli B, Paci A, Ferreira AR, Di Meglio A, Poinsignon V, Bardet A. Serum detection of nonadherence to adjuvant tamoxifen and breast cancer recurrence risk. J Clin Oncol. 2020;38(24):2762–72.
Binkhorst L, Mathijssen RHJ, van Herk-Sukel MPP, Bannink M, Jager A, Wiemer EAC. Unjustified prescribing of CYP2D6 inhibiting SSRIs in women treated with tamoxifen. Breast Cancer Res Treat. 2013;139(3):923–9.
Gong IY, Teft WA, Ly J, Chen Y-H, Alicke B, Kim RB. Determination of clinically therapeutic endoxifen concentrations based on efficacy from human MCF7 breast cancer xenografts. Breast Cancer Res Treat. 2013;139(1):61–9.
Hawse JR, Subramaniam M, Cicek M, Wu X, Gingery A, Grygo SB. Endoxifen’s molecular mechanisms of action are concentration dependent and different than that of other anti-estrogens. PLoS ONE. 2013;8(1):e54613.
Sanchez-Spitman A, Dezentjé V, Swen J, Moes DJAR, Böhringer S, Batman E. Tamoxifen pharmacogenetics and metabolism: results from the prospective CYPTAM study. J Clin Oncol. 2019;37(8):636–46.
Brauch H, Schroth W, Mürdter T, Schwab M. Tamoxifen pharmacogenetics and metabolism: the same is not the same. J Clin Oncol. 2019;37(22):1981–2.
Braal CL, Beijnen JH, Koolen SLW, Oomen-de Hoop E, Steeghs N, Jager A. Relevance of endoxifen concentrations: absence of evidence is not evidence of absence. J Clin Oncol. 2019;37(22):1980–1.
Goetz MP, Suman VJ, Nakamura Y, Kiyotani K, Jordan VC, Ingle JN. Tamoxifen metabolism and breast cancer recurrence: a question unanswered by CYPTAM. J Clin Oncol. 2019;37(22):1982–3.
Guchelaar H-J, Sanchez-Spitman A, Dezentjé V, Böhringer S, Swen J, Neven P. Reply to C.L. Braal et al, H. Brauch et al, and M.P. Goetz et al. J Clin Oncol. 2019;37(22):1984–5.
Stearns V. Reply to H. Brauch et al. J Clin Oncol. 2019;37(22):1986.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by an unrestricted MRACE grant (Erasmus MC, The Netherlands; Grant number 2017-17108).
Conflict of interest
C. Louwrens Braal, Agnes Jager, Esther Oomen–de Hoop, Justin D. Westenberg, Koen M.W.T. Lommen, Peter de Bruijn, Mijntje M. Vastbinder, Quirine C. van Rossum–Schornagel, Martine F. Thijs–Visser, Robbert J. van Alphen, Liesbeth E.M. Struik, Hanneke J.M. Zuetenhorst, Ron H.J. Mathijssen, and Stijn L.W. Koolen have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
The prospective clinical study was approved by the local ethics committee (Erasmus MC, Rotterdam; MEC 17-548) and was registered in the Dutch Trial Registry (NL6918).
Consent
Written informed consent was obtained from all patients participating in the TOTAM study.
Availability of data and material
Available upon reasonable request.
Author contributions
All authors participated in data analysis and interpretation and the writing and editing of the manuscript. Creation of figures and tables: LB, AJ, RM, and SK.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Braal, C.L., Jager, A., Hoop, E.Od. et al. Therapeutic Drug Monitoring of Endoxifen for Tamoxifen Precision Dosing: Feasible in Patients with Hormone-Sensitive Breast Cancer. Clin Pharmacokinet 61, 527–537 (2022). https://doi.org/10.1007/s40262-021-01077-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01077-z