Abstract
Background
Medication use is highly prevalent with advanced age, but clinical studies are rarely conducted in the elderly, leading to limited knowledge regarding age-related pharmacokinetic changes.
Objective
The objective of this study was to investigate which pharmacokinetic parameters determine drug exposure changes in the elderly by conducting virtual clinical trials for ten drugs (midazolam, metoprolol, lisinopril, amlodipine, rivaroxaban, repaglinide, atorvastatin, rosuvastatin, clarithromycin and rifampicin) using our physiologically based pharmacokinetic (PBPK) framework.
Methods
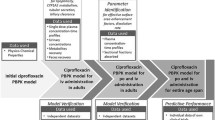
PBPK models for all ten drugs were developed in young adults (20–50 years) following the best practice approach, before predicting pharmacokinetics in the elderly (≥ 65 years) without any modification of drug parameters. A descriptive relationship between age and each investigated pharmacokinetic parameter (peak concentration [Cmax], time to Cmax [tmax], area under the curve [AUC], clearance, volume of distribution, elimination-half-life) was derived using the final PBPK models, and verified with independent clinically observed data from 52 drugs.
Results
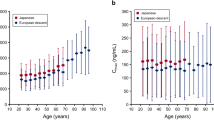
The age-related changes in drug exposure were successfully simulated for all ten drugs. Pharmacokinetic parameters were predicted within 1.25-fold (70%), 1.5-fold (86%) and 2-fold (100%) of clinical data. AUC increased progressively by 0.9% per year throughout adulthood from the age of 20 years, which was explained by decreased clearance, while Cmax, tmax and volume of distribution were not affected by human aging. Additional clinical data of 52 drugs were contained within the estimated variability of the established age-dependent correlations for each pharmacokinetic parameter.
Conclusion
The progressive decrease in hepatic and renal blood flow, as well as glomerular filtration, rate led to a reduced clearance driving exposure changes in the healthy elderly, independent of the drug.









Similar content being viewed by others
References
Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. Washington, DC: United States Census Bureau, Economics and Statistics Administration; 2014.
European Union—Eurostats. People in the EU—population projections; 2017. https://ec.europa.eu/eurostat/statistics-explained/index.php/People_in_the_EU_-_population_projections#Age_dependency_ratios. Cited 6 Feb 2019.
Jaul E, Barron J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health. 2017;5:335–41.
Eurostat. Medicine use statistics; 2014. https://ec.europa.eu/eurostat/statistics-explained/index.php/Medicine_use_statistics. Cited 15 Nov 2018.
US Food and Drug Administration. Diversity in clinical trials; 2018. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm535306.htm. Cited 15 Nov 2018.
Stader F, Siccardi M, Battegay M, Kinvig H, Penny MA, Marzolini C. Repository describing an aging population to inform physiologically based pharmacokinetic models considering anatomical, physiological, and biological age-dependent changes. Clin Pharmacokinet. 2019;58(4):483–501.
Polasek TM, Patel F, Jensen BP, Sorich MJ, Wiese MD, Doogue MP. Predicted metabolic drug clearance with increasing adult age. Br J Clin Pharmacol. 2013;75(4):1019–28.
Burt HJ, Riedmaier AE, Harwood MD, Crewe HK, Gill KL, Neuhoff S. Abundance of hepatic transporters in Caucasians: a meta-analysis. Drug Metab Dispos. 2016;44(10):1550–61.
Schlender J-F, Meyer M, Thelen K, Krauss M, Willmann S, Eissing T, et al. Development of a whole-body physiologically based pharmacokinetic approach to assess the pharmacokinetics of drugs in elderly individuals. Clin Pharmacokinet. 2016;55(12):1573–89.
Chetty M, Johnson TN, Polak S, Salem F, Doki K, Rostami-Hodjegan A. Physiologically based pharmacokinetic modelling to guide drug delivery in older people. Adv Drug Deliv Rev. 2018;135:85–96.
Stader F, Penny MA, Siccardi M, Marzolini C. A comprehensive framework for physiologically based pharmacokinetic modelling in Matlab®. CPT Pharmacomet Syst Pharmacol. 2019;8(7):444–59.
Rowland-Yeo K, Jamei M, Rostami-Hodjegan A. Predicting drug–drug interactions: application of physiologically based pharmacokinetic models under a systems biology approach. Expert Rev Clin Pharmacol. 2013;6(2):143–57.
Chetty M, Rose RH, Abduljalil K, Patel N, Lu G, Cain T, et al. Applications of linking PBPK and PD models to predict the impact of genotypic variability, formulation differences, differences in target binding capacity and target site drug concentrations on drug responses and variability. Front Pharmacol. 2014;5(258):1–14.
Mukherjee D, Zha J, Menon RM, Shebley M. Guiding dose adjustment of amlodipine after co-administration with ritonavir containing regimens using a physiologically-based pharmacokinetic/pharmacodynamic model. J Pharmacokinet Pharmacodyn. 2018;45(3):443–56.
Marzolini C, Rajoli R, Battegay M, Elzi L, Back D, Siccardi M. Physiologically based pharmacokinetic modeling to predict drug–drug interactions with efavirenz involving simultaneous inducing and inhibitory effects on cytochromes. Clin Pharmacokinet. 2017;56(4):409–20.
Zhang T. Physiologically based pharmacokinetic modeling of disposition and drug–drug interactions for atorvastatin and its metabolites. Eur J Pharm Sci. 2015;77:216–29.
Jamei M, Bajot F, Neuhoff S, Barter Z, Yang J, Rostami-Hodjegan A, et al. A mechanistic framework for in vitro–in vivo extrapolation of liver membrane transporters: prediction of drug–drug interaction between rosuvastatin and cyclosporine. Clin Pharmacokinet. 2014;53(1):73–87.
Rowland-Yeo K, Walsky R, Jamei M, Rostami-Hodjegan A, Tucker G. Prediction of time-dependent CYP3A4 drug–drug interactions by physiologically based pharmacokinetic modelling: impact of inactivation parameters and enzyme turnover. Eur J Pharm Sci. 2011;43(3):160–73.
Varma MV, Lai Y, Kimoto E, Goosen TC, El-Kattan AF, Kumar V. Mechanistic modeling to predict the transporter-and enzyme-mediated drug–drug interactions of repaglinide. Pharm Res. 2013;30(4):1188–99.
Faulkner J, McGibney D, Chasseaud L, Perry J, Taylor I. The pharmacokinetics of amlodipine in healthy volunteers after single intravenous and oral doses and after 14 repeated oral doses given once daily. Br J Clin Pharmacol. 1986;22(1):21–5.
Stader F, Kinvig H, Battegay M, Khoo S, Owen A, Siccardi M, et al. Analysis of clinical drug–drug interaction data to predict uncharacterized interaction magnitudes between antiretroviral drugs and co-medications. Antimicrob Agents Chemother. 2018;62(7):1–12.
Zhu Y, Wang F, Li Q, Zhu M, Du A, Tang W, et al. Amlodipine metabolism in human liver microsomes and roles of CYP3A4/5 in the dihydropyridine dehydrogenation. Drug Metab Dispos. 2014;42(2):245–9.
Varma MV, Lai Y, Feng B, Litchfield J, Goosen TC, Bergman A. Physiologically based modeling of pravastatin transporter-mediated hepatobiliary disposition and drug–drug interactions. Pharm Res. 2012;29(10):2860–73.
Almond LM, Mukadam S, Gardner I, Okialda K, Wong S, Hatley O, et al. Prediction of drug–drug interactions arising from CYP3A induction using a physiologically based dynamic model. Drug Metab Dispos. 2016;44(6):821–32.
Ke A, Barter Z, Rowland-Yeo K, Almond L. Towards a best practice approach in PBPK modeling: case example of developing a unified efavirenz model accounting for induction of CYPs 3A4 and 2B6. CPT Pharmacomet Syst Pharmacol. 2016;5(7):367–76.
Kendall M, Brown D, Yates R. Plasma metoprolol concentrations in young, old and hypertensive subjects. Br J Clin Pharmacol. 1977;4(4):497–9.
Gautam P, Vargas E, Lye M. Pharmacokinetics of lisinopril (MK521) in healthy young and elderly subjects and in elderly patients with cardiac failure. J Pharm Pharmacol. 1987;39(11):929–31.
Gomez HJ, Cirillo VJ, Moncloa F. The clinical pharmacology of lisinopril. J Cardiovasc Pharmacol. 1987;9:S27–34.
Ulm E, Hichens M, Gomez H, Till A, Hand E, Vassil T, et al. Enalapril maleate and a lysine analogue (MK-521): disposition in man. Br J Clin Pharmacol. 1982;14(3):357–62.
Sagirli O, Ersoy L. An HPLC method for the determination of lisinopril in human plasma and urine with fluorescence detection. J Chromatogr B. 2004;809(1):159–65.
Acocella G, Pagani V, Marchetti M, Baroni G, Nicolis F. Kinetic studies on rifampicin. I. Serum concentration analysis in subjects treated with different oral doses over a period of two weeks. Chemotherapy. 1971;16(6):356–70.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(13):1–10.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135–59.
Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology. 1984;61(1):27–35.
Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. Complex drug interactions of HIV protease inhibitors 1: inactivation, induction and inhibition of cytochrome P450 3A by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39(6):1070–8.
Quarterman C, Kendall M, Jack D. The effect of age on the pharmacokinetics of metoprolol and its metabolites. Br J Clin Pharmacol. 1981;11(3):287–94.
Obach RS, Lombardo F, Waters NJ. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab Dispos. 2008;36(7):1385–405.
Abernethy DR, Gutkowska J, Lambert MD. Amlodipine in elderly hypertensive patients: pharmacokinetics and pharmacodynamics. J Cardiovasc Pharmacol. 1988;12:S67–71.
Kudo T, Goda H, Yokosuka Y, Tanaka R, Komatsu S, Ito K. Estimation of the contribution of CYP2C8 and CYP3A4 in repaglinide metabolism by human liver microsomes under various buffer conditions. J Pharm Sci. 2017;106(9):2847–52.
Hatorp V, Huang W-C, Strange P. Repaglinide pharmacokinetics in healthy young adult and elderly subjects. Clin Ther. 1999;21(4):702–10.
Gibson DM, Bron NJ, Richens MA, Hounslow NJ, Sedman AJ, Whitfield LR. Effect of age and gender on pharmacokinetics of atorvastatin in humans. J Clin Pharmacol. 1996;36(3):242–6.
Rodvold KA. Clinical pharmacokinetics of clarithromycin. Clin Pharmacokinet. 1999;37(5):385–98.
Acocella G. Clinical pharmacokinetics of rifampicin. Clin Pharmacokinet. 1978;3(2):108–27.
Regårdh C, Landahl S, Larsson M, Lundborg P, Steen B, Hoffmann K-J, et al. Pharmacokinetics of metoprolol and its metabolite α-OH-metoprolol in healthy, non-smoking, elderly individuals. Eur J Clin Pharmacol. 1983;24(2):221–6.
Beermann B. Pharmacokinetics of lisinopril. Am J Med. 1988;85(3):25–30.
Jamei M, Dickinson GL, Rostami-Hodjegan A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of ‘bottom-up’ vs ‘top-down’ recognition of covariates. Drug Metab Pharmacokinet. 2009;24(1):53–75.
Lennernäs H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42(13):1141–60.
Vestal RE, McGuire EA, Tobin JD, Andres R, Norris AH, Mezey E. Aging and ethanol metabolism. Clin Pharmacol Ther. 1977;21(3):343–54.
Redolfi A, Borgogelli E, Lodola E. Blood level of cimetidine in relation to age. Eur J Clin Pharmacol. 1979;15(4):257–61.
Abernethy D, Greenblatt D, Shader R. Imipramine and desipramine disposition in the elderly. J Pharmacol Exp Ther. 1985;232(1):183–8.
Castleden CM, George CF. The effect of ageing on the hepatic clearance of propranolol. Br J Clin Pharmacol. 1979;7(1):49–54.
Jaillon P, Gardin M, Lecocq B, Richard M, Meignan S, Blondel Y, et al. Pharmacokinetics of nalbuphine in infants, young healthy volunteers, and elderly patients. Clin Pharmacol Ther. 1989;46(2):226–33.
Rho JP, Jones A, Woo M, Castle S, Smith K, Bawdon RE, et al. Single-dose pharmacokinetics of intravenous ampicillin plus sulbactam in healthy elderly and young adult subjects. J Antimicrob Chemother. 1989;24(4):573–80.
Cusack B, Kelly J, O’Malley K, Noel J, Lavan J, Horgan J. Digoxin in the elderly: pharmacokinetic consequences of old age. Clin Pharmacol Ther. 1979;25(6):772–6.
Achour B, Russell MR, Barber J, Rostami-Hodjegan A. Simultaneous quantification of the abundance of several cytochrome P450 and uridine 5′-diphospho-glucuronosyltransferase enzymes in human liver microsomes using multiplexed targeted proteomics. Drug Metab Dispos. 2014;42(4):500–10.
Parkinson A, Mudra D, Johnson C, Dwyer A, Carroll K. The effects of gender, age, ethnicity, and liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol. 2004;199(3):193–209.
Simon T, Becquemont L, Hamon B, Nouyrigat E, Chodjania Y, Poirier J, et al. Variability of cytochrome P450 1A2 activity over time in young and elderly healthy volunteers. Br J Clin Pharmacol. 2001;52(5):601–4.
Vestal R, Wood A, Branch R, Shand D, Wilkinson G. Effects of age and cigarette smoking on propranolol disposition. Clin Pharmacol Ther. 1979;26(1):8–15.
Musso CG, Oreopoulos DG. Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 2011;119(Suppl. 1):1–5.
Hockings N, Ajayi A, Reid J. Age and the pharmacokinetics of angiotensin converting enzyme inhibitors enalapril and enalaprilat. Br J Clin Pharmacol. 1986;21(4):341–8.
Faulkner R, Bohaychuk W, Lanc R, Haynes J, Desjardins R, Yacobi A, et al. Pharmacokinetics of cefixime in the young and elderly. J Antimicrob Chemother. 1988;21(6):787–94.
Kubitza D, Becka M, Roth A, Mueck W. The influence of age and gender on the pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor. J Clin Pharmacol. 2013;53(3):249–55.
Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143–57.
Rowland Yeo K, Aarabi M, Jamei M, Rostami-Hodjegan A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Rev Clin Pharmacol. 2011;4(2):261–74.
Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49(3):189–206.
Rodighiero V. Effects of cardiovascular disease on pharmacokinetics. Cardiovasc Drugs Ther. 1989;3(5):711–30.
Wooten JM. Pharmacotherapy considerations in elderly adults. South Med J. 2012;105(8):437–45.
Albrecht S, Ihmsen H, Hering W, Geisslinger G, Dingemanse J, Schwilden H, et al. The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. Clin Pharmacol Ther. 1999;65(6):630–9.
Goa KL, Balfour JA, Zuanetti G. Lisinopril. A review of its pharmacology and clinical efficacy in the early management of acute myocardial infarction. Drugs. 1996;52(4):564–88.
Leenen FH, Coletta E. Pharmacokinetic and antihypertensive profile of amlodipine and felodipine-ER in younger versus older patients with hypertension. J Cardiovasc Pharmacol. 2010;56(6):669–75.
Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78(4):412–21.
Kubitza D, Becka M, Roth A, Mueck W. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin. 2008;24(10):2757–65.
Szadkowska I, Stanczyk A, Aronow WS, Kowalski J, Pawlicki L, Ahmed A, et al. Statin therapy in the elderly: a review. Arch Gerontol Geriatr. 2010;50(1):114–8.
Vass M, Hendriksen C. Medication for older people. Z Gerontol Geriatr. 2005;38(3):190–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Swiss National Foundation (Grant number 166204), the OPO Foundation, and the Isaac Dreyfus Foundation. Melissa A. Penny was additionally supported by the Swiss National Foundation Professorship (PP00P3 170702).
Conflict of interest
Felix Stader, Hannah Kinvig, Melissa A. Penny, Manuel Battegay, Marco Siccardi, and Catia Marzolini have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stader, F., Kinvig, H., Penny, M.A. et al. Physiologically Based Pharmacokinetic Modelling to Identify Pharmacokinetic Parameters Driving Drug Exposure Changes in the Elderly. Clin Pharmacokinet 59, 383–401 (2020). https://doi.org/10.1007/s40262-019-00822-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00822-9