Abstract
Introduction
Bruton tyrosine kinase (BTK) is a key component of B-cell receptor signalling, critical for cell proliferation. Acalabrutinib, a selective, covalent BTK inhibitor, recently received an accelerated approval in relapsed/refractory mantle cell lymphoma. This analysis characterized the population pharmacokinetics (PK) of acalabrutinib and its metabolite ACP-5862.
Methods
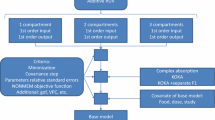
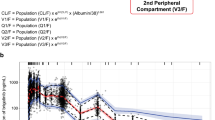
Data were obtained from six phase I/II trials in adult patients with B-cell malignancy and seven phase I trials in healthy volunteers. Pooled concentration-time data, at dose levels ranging from 15 to 400 mg, were analysed using non-linear mixed-effects modelling. Base model parameters were scaled with body weight and normalized to 70 kg (fixed exponents: 0.75 and 1 for clearance and volumes, respectively). A full covariate approach was used to evaluate any relevant effects of dose, health group/disease status, hepatic and renal impairment, use of acid-reducing agents, race and sex.
Results
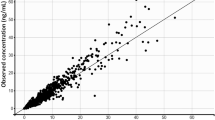
A total of 11,196 acalabrutinib and 1068 ACP-5862 concentration-time samples were available. The PK of both analytes were well described using two-compartment disposition models. Acalabrutinib absorption was characterized using sequential zero- and first-order constants and a lag time. Apparent clearance (CL/F) of acalabrutinib was 169 L/h (95% CI 159–175). Relative to the 100 mg dose group, the 15 and 400 mg dose groups showed a 1.44-fold higher and 0.77-fold lower CL/F, respectively. The clearance for ACP-5862 was 21.9 L/h (95% CI 19.5–24.0). The fraction metabolized was fixed to 0.4. The central and peripheral volumes of distribution were 33.1 L (95% CI 24.4–41.0) and 226 L (95% CI 149–305) for acalabrutinib, and 38.5 L (95% CI 31.6–49.2) and 38.4 L (95% CI 32.3–47.9) for ACP-5862. None of the investigated covariates led to clinically meaningful changes in exposure.
Conclusion
The PK of acalabrutinib and its metabolite ACP-5862 were adequately characterized. Acalabrutinib CL/F decreased with increasing dose, but the trend was small over the 75–250 mg range. No dose adjustment was necessary for intrinsic or extrinsic covariates.



Similar content being viewed by others
References
Küppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–62.
Shaffer AL, Rosenwald A, Staudt LM. Lymphoid malignancies: the dark side of B-cell differentiation. Nat Rev Immunol. 2002;2:920–32.
Gayko U, Fung M, Clow F, Sun S, Faust E, Price S, et al. Development of the Bruton’s tyrosine kinase inhibitor ibrutinib for B cell malignancies. Ann N Y Acad Sci. 2015;1358:82–94.
Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14:219–32 (Nature Publishing Group).
Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, et al. Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase (BTK) inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363:240–52.
Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2017;6736:1–9.
US FDA. Calquence (acalabrutinib) Label. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/210259s000lbl.pdf. Accessed 18 Sept 2018.
Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–32.
Edlund H, Andrew M, Jin F, Patel P, Masson E, Slatter JG, et al. Population Pharmacokinetics of Bruton Tyrosine Kinase Inhibitor, Acalabrutinib, in Healthy Volunteers and Patients with B-Cell Malignancies. Blood. 2017;130(Suppl 1):4997.
Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35:401–21.
Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development—Part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacomet Syst Pharmacol. 2013;2:e38.
Al-Huniti N, Chapel S, Xu H, Bui KH, Sostek M. Population pharmacokinetics of naloxegol in a population of 1247 healthy subjects and patients. Br J Clin Pharmacol. 2016;81:89–100.
Ravva P, Gastonguay MR, Tensfeldt TG, Faessel HM. Population pharmacokinetic analysis of varenicline in adult smokers. Br J Clin Pharmacol. 2009;68:669–81.
Jin F, Yin M, Mandava V, Edlund H, Andrew M, Patel P, et al. Exposure-response of the Bruton’s tyrosine kinase inhibitor, acalabrutinib in the treatment of hematologic malignancies. Blood. 2017;130(Suppl 1):1268.
R Core Team. R: a language and environment for statistical computing. Vienna; 2015. http://www.r-project.org/.
Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM user’s guides (1989–2009). Ellicott City: Icon Development Solutions; 2009.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN): a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75:85–94.
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34:711–26.
Yates JW, Jones RO, Walker M, Cheung SA. Structural identifiability and indistinguishability of compartmental models. Expert Opin Drug Metab Toxicol. 2009;5:295–302.
Rowland M, Tozer TN. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. 4th ed. Philadelphia: Lippincott, Williams & Wilkins; 2011.
Acknowledgements
Karthick Vishwanathan (AstraZeneca), Eric Masson (Biogen, previously at AstraZeneca), Feng Jin (Theravance, previously at Acerta Pharma), Jennifer Juntado (Acerta Pharma), Ming Yin (Acerta Pharma).
Author information
Authors and Affiliations
Contributions
H. Edlund designed and performed the research, analysed the PK data (parent) and drafted the manuscript; S.K. Lee designed and performed the research, analysed the PK data (metabolite) and edited the manuscript; and M.A. Andrew, J.G. Slatter, S. Aksenov, and N. Al-Huniti designed the research and edited the manuscript.
Corresponding author
Ethics declarations
Funding
The underlying clinical studies were sponsored by Acerta Pharma (a member of the AstraZeneca group).
Conflict of interest
Helena Edlund, Sun Ku Lee, Marilee A. Andrew, J. Greg Slatter, Sergey Aksenov and Nidal Al-Huniti were all employed by Acerta Pharma or AstraZeneca at the time this work was conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Edlund, H., Lee, S.K., Andrew, M.A. et al. Population Pharmacokinetics of the BTK Inhibitor Acalabrutinib and its Active Metabolite in Healthy Volunteers and Patients with B-Cell Malignancies. Clin Pharmacokinet 58, 659–672 (2019). https://doi.org/10.1007/s40262-018-0725-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-018-0725-7