Abstract
Objective
The aim of this study was to understand the influence of disease and patient characteristics on exposure to daratumumab, an immunoglobulin Gκ (IgGκ) monoclonal antibody, and clinical outcomes in relapsed or refractory multiple myeloma (MM).
Patients and Methods
Baseline myeloma type, albumin levels, renal/hepatic function, age, sex, race, weight, Eastern Cooperative Oncology Group (ECOG) status, refractory status, and number of prior therapies were evaluated using data from two clinical studies—GEN501 (N = 104) and SIRIUS (N = 124).
Results
Daratumumab clearance was approximately 110% higher in IgG myeloma patients than non-IgG myeloma patients, leading to significantly lower exposure in IgG myeloma patients based on maximum trough serum concentrations (p < 0.0001). However, the overall response rate was similar for IgG and non-IgG myeloma patients (odds ratio 1.08, 95% confidence interval 0.54–2.17, p = 0.82). For a given exposure, the drug effect was significantly higher (approximately two times) in IgG versus non-IgG patients (p = 0.03). The influence of other patient and disease characteristics on daratumumab exposure was minimal and no significant effect on efficacy was observed (p ≥ 0.1). The incidences of infections and overall grade 3 or higher adverse events in subpopulations were generally consistent with that of the overall population.
Conclusion
Due to competition with the MM-produced IgG M-protein for neonatal Fc receptor protection from clearance, IgG-based monoclonal antibodies in general may have significantly higher clearance and lower concentrations in IgG MM patients compared with non-IgG MM patients. Careful evaluation of the impact of exposure and patient and disease characteristics on safety and efficacy is warranted for all IgG-based monoclonal antibodies used in MM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Generally, immunoglobulin G (IgG) myeloma patients may have greater clearance of therapeutic antibodies, leading to lower exposure versus non-IgG patients. |
For daratumumab, IgG patients may show increased sensitivity, attaining similar efficacy at lower concentrations versus non-IgG patients. |
1 Introduction
Daratumumab, a human immunoglobulin G1κ (IgG1κ) monoclonal antibody (mAb), binds with high affinity to CD38, which is ubiquitously expressed on myeloma cells [1, 2]. Daratumumab induces CD38 immune-mediated activities, apoptosis, and modulation of CD38 enzymatic activity [3,4,5,6], and has immunomodulatory effects that minimize the functions of CD38+ immunosuppressive cells, expand T-cell numbers, and increase T-cell clonality [7]. Daratumumab has been shown to provide clinical benefit for the treatment of multiple myeloma (MM) in patients with one or more prior line of therapy [8, 9]. It received approval from the US FDA and European Medicines Agency (EMA) for use as monotherapy in heavily treated patients with relapsed or refractory MM [10, 11], and was recently approved in combination with bortezomib and dexamethasone, or lenalidomide and dexamethasone, in MM patients who have received at least one prior therapy [12].
The malignant cells of MM patients can produce excessive amounts of monoclonal Ig (so-called myeloma protein or M-protein), including IgG [13], which may affect IgG-based mAb treatments such as daratumumab. Excessive endogenous IgG M-protein can cause elevated clearance of IgG-based mAbs due to the competition between endogenous disease-produced IgG and exogenous therapeutic IgG for neonatal Fc receptor (FcRn)–mediated IgG protection [14]. Therefore, IgG myeloma patients may have lower exposure to daratumumab compared with non-IgG myeloma patients. In addition, it was of interest to investigate whether the predicted difference in daratumumab exposure between IgG and non-IgG patients might impact its efficacy and safety.
Additionally, albumin, a factor used in the International Staging System (ISS) for defining prognosis in myeloma and which is also a substrate of FcRn, can also potentially affect exposure to mAbs and the drug effect in MM patients at different ISS classifications [15]. Furthermore, up to 50% of MM patients experience a decrease in creatinine clearance [16]. It remains unknown how these disease characteristics of MM patients may affect the clinical pharmacology, efficacy, and safety of daratumumab.
In two studies of daratumumab monotherapy (GEN501 and SIRIUS), deep and durable responses were achieved in patients with heavily treated relapsed and refractory MM [17, 18]. Data collected from patients with pharmacokinetic (PK) samples from these two phase II studies provided a unique opportunity to understand the influence of the mechanism-based interaction between a therapeutic mAb and IgG M-protein, disease-induced renal insufficiency, and albumin levels on exposure and, consequently, the clinical outcomes of daratumumab-treated patients in relapsed or refractory MM. In addition, we also explored the impact of other disease and patient characteristics, such as body weight, age, sex, race, hepatic function, Eastern Cooperative Oncology Group (ECOG) status, refractory status, and number of prior therapies on daratumumab PK, efficacy, and safety.
2 Methods
2.1 Patients and Study Design
This exploratory analysis was conducted using combined data from relapsed/refractory MM patients enrolled in two clinical studies: GEN501 (NCT00574288) and SIRIUS (MMY2002; NCT01985126). Study designs and treatment schedules have been described in depth elsewhere [17, 18]. Briefly, GEN501 was an open-label, phase I/II study [17]. In the dose-escalation phase, intravenous daratumumab doses ranged from 0.005 to 24 mg/kg, while in the dose-extension phase, daratumumab 8 or 16 mg/kg was administered intravenously once every week for 8 weeks, every 2 weeks for 16 weeks, and every 4 weeks thereafter. SIRIUS was an open-label, multicenter, phase II study in which patients were initially randomized to intravenous daratumumab 8 or 16 mg/kg, with patients subsequently receiving 16 mg/kg once every week for 8 weeks, every 2 weeks for 16 weeks, and every 4 weeks thereafter [18]. Eligibility criteria in both studies included patients 18 years of age and older with documented myeloma and an ECOG performance status ≤2. GEN501 enrolled patients who had relapsed from or were refractory to two or more prior lines of treatment, including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), chemotherapy, and autologous stem cell transplantation [17], whereas SIRIUS enrolled patients who had received three or more prior therapies, including a PI and an IMiD, or who were double refractory to both a PI and an IMiD [18]. Based on these data, 16 mg/kg once every week for 8 weeks, every 2 weeks for 16 weeks, and every 4 weeks thereafter was established as the recommended dosing schedule for daratumumab for clinical use.
2.2 Disease and Patient Characteristics
Disease factors included type of myeloma (IgG vs. non-IgG), number of prior lines of therapy, refractory status, and ECOG performance status at baseline; patient demographics included age, body weight, sex, race, renal and hepatic function, and albumin levels. Hepatic impairment was defined using the National Cancer Institute criteria of hepatic dysfunction [19].
2.3 Statistical Analysis
The influence of disease and patient characteristics on daratumumab PK was evaluated using a two-compartment model with parallel linear (i.e. non-specific protein catabolism) and non-linear (i.e. target CD38-mediated) eliminations [20]. The daratumumab serum concentrations used for PK analyses were measured by a validated enzyme-linked immunosorbent assay (BioAnalytical Research Corporation Global Central Laboratory, Ghent, Belgium; Janssen Research & Development, LLC, Spring House, PA, USA). This assay has a lower limit of quantification of 0.2 µg/mL. A subgroup analysis on the model-predicted maximum trough concentration at the end of once-weekly dosing on day 1 of cycle 3 was conducted to quantify the influence of individual disease and patient characteristics on exposure to daratumumab. Maximal trough concentration was found to have the strongest correlation with efficacy endpoints following daratumumab treatment [20].
The influence of disease and patient characteristics on clinical efficacy was evaluated by subgroup analyses on the overall response rate (ORR) [17, 18]. Logistic regression modeling of ORR was performed in R version 3.2.2. Individual disease and patient characteristics were included in the univariate models and a multivariate model that included all of the investigated factors was also constructed. In addition, logistic regression models were developed to examine the interaction between daratumumab exposure and type of myeloma, and to quantify the relationship between drug concentration and ORR separately in patients with IgG and non-IgG MM. Subgroup analyses were performed for overall grade 3 or higher adverse events (AEs) and infection events (all grades and grade 3 or higher).
3 Results
3.1 Patient and Disease Characteristics
Together, the GEN501 and SIRIUS studies included 228 patients, of whom 151 received the recommended dose of daratumumab 16 mg/kg. PK samples were collected from 223 patients. Electronic supplementary Table 1 presents baseline disease and patient characteristics for each study individually, as well as for the combined population.
3.2 Influence of Patient and Disease Characteristics on the Pharmacokinetics of Daratumumab
Electronic supplementary Fig. 1 shows the daratumumab trough serum concentration versus time profile for patients receiving daratumumab 16 mg/kg, stratified by disease and patient characteristics. With the exception of type of myeloma, no visible separation in concentration-time profiles was observed for the other disease and patient characteristics. For type of myeloma, daratumumab serum concentrations were lower in IgG myeloma patients compared with non-IgG patients (Fig. 1a).
Trough concentration versus time profile for patients receiving daratumumab 16 mg/kg in the GEN501 and SIRIUS studies, stratified by type of myeloma (IgG vs. non-IgG). a The trend line and band represent the LOESS regression line and the associated 95% confidence intervals, respectively. b Box plot of individual linear clearances for IgG myeloma patients and non-IgG myeloma patients. Linear clearance refers to the non-specific clearance of monoclonal antibodies due to protein catabolism. IgG immunoglobulin G
The linear clearance of daratumumab was significantly (110%; p < 0.0001) higher in patients with IgG MM (median value of 0.0115 L/h) than in patients with non-IgG disease (median value of 0.00549 L/h; Fig. 1b). The predicted trough serum concentrations on day 1 of cycle 3 in non-IgG myeloma patients were approximately 70% higher than those of IgG myeloma patients (Fig. 2).
Forest plot of subgroup analyses to evaluate the influence of disease and patient characteristics on predicted maximal pre-infusion (trough) concentration. Solid blue circles represent means, and error bars represent 95% CIs. The dashed line represents the mean value (530.65) in the overall population. CI confidence interval, IgG immunoglobulin G, ECOG Eastern Cooperative Oncology Group
In addition, in IgG myeloma patients, the linear clearance was significantly correlated with baseline IgG levels (p < 0.0001; electronic supplementary Fig. 2); however, a large variability in clearance was observed.
Both linear clearance and central volume of distribution (V1) of daratumumab significantly increased with increasing body weight (p < 0.0001). Doubling of body weight was associated with a 65 and 50% increase in linear clearance and V1, respectively. However, exposure to daratumumab was generally consistent over a range of different body weights after administration on a mg/kg basis (Fig. 2).
Baseline albumin concentration had a statistically significant correlation with linear clearance of daratumumab (p < 0.0001), although the observed concentrations appear to be similar, regardless of baseline albumin levels (electronic supplementary Fig. 1). The predicted trough serum concentration in patients with abnormal baseline albumin was 26% lower than that of patients with normal baseline albumin. Daratumumab exposure was similar across subpopulations stratified by other demographic or disease characteristics, including renal function.
3.3 Influence of Patient and Disease Characteristics on the Efficacy of Daratumumab
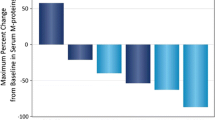
Subgroup analyses of ORR in the patients who received daratumumab 16 mg/kg are presented in Fig. 3. ORR was similar for IgG myeloma patients (32%; 95% confidence interval [CI] 22.5–43.3%) and non-IgG myeloma patients (30.3%; 95% CI 21.0–41.4%). By univariate and multivariate regression analyses, the odds ratios (ORs) for IgG and non-IgG patients were 1.08 (95% CI 0.54–2.17, p = 0.82) and 1.12 (95% CI 0.54–2.36, p = 0.76), respectively (Table 1). The ORR across the subpopulations for other patient and disease characteristics, including renal and hepatic impairment, was also consistent with the ORR in the overall patient population (Fig. 3). None of these investigated characteristics were statistically significant in either univariate or multivariate analyses (p ≥ 0.1). Analyses of maximum relative changes in M-protein from baseline also confirmed similar changes in M-protein between patients with IgG and non-IgG MM (electronic supplementary Fig. 3).
ORR in patients receiving daratumumab 16 mg/kg in the GEN501 and SIRIUS studies, stratified by disease and patient characteristics. Solid blue circles represent means, and error bars represent 95% CIs. ORR overall response rate, CI confidence interval, IgG immunoglobulin G, ECOG Eastern Cooperative Oncology Group
3.4 Evaluation of Daratumumab Drug Effect in Immunoglobulin (Ig) G and Non-IgG Myeloma Patients
The effect of daratumumab was highly significant in the overall population of patients receiving 16 mg/kg (p < 0.0001), as well as in patients with IgG (p < 0.0001) and non-IgG MM (p = 0.004) [Table 2]. The estimated concentration effect on ORR in patients with non-IgG MM was significantly lower compared with that of patients with IgG myeloma (p = 0.03); the log OR of the concentration effect (0.32, 95% CI 0.12–0.56, per 100 µg/mL increase in maximum trough concentration) was approximately half that of patients with non-IgG MM (0.76, 95% CI 0.46–1.14). The increase in OR was estimated to be 37% per 100 µg/mL increase in maximum trough serum concentration in non-IgG myeloma patients, whereas a 114% increase in OR was observed in IgG myeloma patients (Table 2). After adjusting for daratumumab serum concentration and the interaction between daratumumab serum concentration and type of myeloma, the effect of type of myeloma remained non-significant (p = 0.5), although the possibility that the number of patients was too low to detect a difference in response cannot be excluded.
3.5 Influence of Patient and Disease Characteristics on Safety Following Daratumumab Administration
Table 3 summarizes the incidence of infections (any grade or grade 3 or higher) and overall grade 3 or higher AEs among different patient subgroups. Forty-five of 75 patients with IgG MM (60%) had an infection (any grade), while 38 of 76 patients with non-IgG MM (50%) experienced an infection (any grade). Thirteen percent and 7% of patients with IgG and non-IgG MM, respectively, experienced grade 3 or higher infections. These results were similar to the infection rate in the overall population, where approximately 55% of patients had infections of any grade and approximately 10% had grade 3 or higher infections. Similarly, the rate of grade 3 or higher AEs was comparable between patients with IgG and non-IgG MM (59 and 51%, respectively) and was consistent with the rate observed in the overall population (55%). Double refractory patients appeared to experience more grade 3 or higher AEs than patients who were not double refractory. The rate of grade 3 or higher AEs and the rate of infections (any grade or grade 3 or higher) was generally consistent with the rate observed in the overall population when stratified by the other investigated patient and disease characteristics.
4 Discussion
Many factors may affect therapeutic mAb distribution [21]. MM patients exhibit several unique disease characteristics, such as excessive production of IgG, and reduced albumin levels and renal dysfunction as disease progresses. These characteristics could potentially interact with disposition of mAbs and impact clinical outcomes. Our research is the first report to reveal significantly lower concentrations of a therapeutic mAb (i.e. daratumumab) in patients with IgG MM compared with non-IgG MM. Overall, these results suggest that IgG patients may be more sensitive to daratumumab at a given exposure compared with non-IgG patients. Therefore, even though IgG myeloma patients had significantly lower daratumumab concentrations compared with non-IgG myeloma patients, the clinical efficacy of daratumumab was comparable between the two groups.
In addition, there was a significant correlation between baseline IgG M-protein levels and linear clearance of daratumumab, which represents non-specific clearance of mAbs due to protein catabolism (electronic supplementary Fig. 1). It is likely that this effect was due to the unique interaction between IgG clearance and the excessive amount of monoclonal M-protein produced in MM patients. The levels of M-protein in MM patients are usually in the g/L range, which is at least 10 times higher than most therapeutic mAb concentrations [22]. FcRn, expressed on various organs, including the reticuloendothelial system [14, 23], has been shown to protect IgG or IgG-based monoclonal antibodies from degradation due to intracellular catabolism, resulting in low clearance and long half-lives for these classes of mAbs in serum [14]. The lower daratumumab exposure observed in patients with IgG MM compared with non-IgG MM was likely due to the competition between the high concentration of disease-produced IgG M-protein secreted by myeloma cells and daratumumab, an IgG antibody, for FcRn protection from elimination. Since most existing therapeutic antibodies are of the IgG isotype, the influence of type of myeloma and IgG M-protein on mAb exposure should be considered for daratumumab and other therapeutic antibodies used in MM patients [24].
The lower daratumumab exposure observed in patients with IgG myeloma raises the question of whether higher doses of daratumumab might improve efficacy in these patients. When considering this question, it is important to note that exposure is not the only factor that may drive response to daratumumab; density of CD38 receptors, receptor occupancy, and disease severity, among other cellular or physiologic conditions, may impact outcomes. To date, published data and ongoing clinical studies with higher doses do not support adjustment to the recommended daratumumab dose in patients with IgG myeloma. PK modeling indicates that increasing the intravenous daratumumab dose beyond the threshold of maximal effect, which is reached in the majority of patients treated with the recommended 16 mg/kg dose, would not result in a benefit to efficacy [25]. This is consistent with preliminary data in patients with IgG myeloma from an ongoing clinical study with a higher dose (NCT02519452). Current data show that at a dose of 16 mg/kg, ORRs in IgG patients were similar to those seen in non-IgG patients, suggesting that, compared with non-IgG patients, IgG patients may need lower concentrations to attain similar clinical response rates (i.e. they have a lower threshold of effective concentration). Future studies are planned in which patients will be stratified based on MM type; data from those studies are likely to inform the need for additional evaluation of the interaction between daratumumab exposure, outcomes, and IgG.
FcRn also binds to and protects albumin from protein catabolism [15, 22]. A higher albumin concentration indicates the presence of higher FcRn levels, which, in turn, may also increase the protection of daratumumab from protein catabolism. Baseline albumin concentration had a statistically significant effect on linear clearance of daratumumab; however, the magnitude of the difference between normal and abnormal albumin levels on daratumumab exposure was relatively small (26%). In addition, although the ORR in patients with normal albumin levels appeared to be higher than in patients with abnormal albumin levels, the ORR in both groups was generally consistent with that of the overall population and the CIs overlapped each other. Logistic regression also showed that albumin levels did not have a statistically significant effect on ORR when albumin was evaluated as a continuous variable. The lower ORR in patients with abnormal albumin [26] may be explained by severity of disease: as albumin is a factor used to stage MM, patients with more advanced disease have lower albumin levels and tend to be less responsive to daratumumab treatment. Furthermore, patients with abnormal albumin also tended to have a higher rate of grade 3 or higher AEs, despite a lower concentration of daratumumab. Thus, increasing the daratumumab dose in patients with abnormal albumin in order to improve ORR is unlikely to improve the overall risk–benefit profile.
Proliferation of M-protein has several deleterious effects on many organs, including the kidneys [13]. Two-thirds of patients evaluated in this analysis had some degree of pre-existing renal impairment. The analysis presented here demonstrates that renal function did not have a significant effect on daratumumab clearance and exposure and also had a minimal impact on efficacy and safety. The trend toward a higher rate of overall grade 3 or higher AEs in patients with renal impairment was likely a result of the large proportion of double refractory patients included in this patient population.
Similarly, mild hepatic impairment did not have a significant impact on the exposure and clinical outcomes for daratumumab. The seemingly lower exposure in patients with mild hepatic impairment (approximately 20%) [Fig. 2] may have been the result of the unbalanced distribution of patients with IgG MM to groups with mild hepatic impairment (65%; n = 24 at 16 mg/kg) versus normal hepatic function (46%; n = 127 at 16 mg/kg). The ORR was almost identical for patients with mild hepatic impairment (33%; 95% CI 16.9–53.2%) and normal hepatic function (31%; 95% CI 23.1–39.1%) at 16 mg/kg. The incidence of overall grade 3 or higher AEs and infections (3 or higher, or any grade) in both groups was consistent with that of the overall population.
Natural killer (NK) cells regulate immune activity, thus providing protection against infection [27]. Because CD38 is expressed on NK cells, daratumumab tends to reduce NK cells in a dose-dependent manner [28]. Thus, the possibility that daratumumab treatment might result in different rates of infection in subgroups was examined. The present analysis demonstrates that the incidence of infection was consistent among the subgroups for all investigated disease and patient characteristics, including type of myeloma. Our previous research demonstrated that there was a weak trend toward higher rates of infection of any grade with greater daratumumab exposure, but this observation did not reach statistical significance and a similar trend was not observed for grade 3 or higher infections. The consistent infection rates may be partly explained by the lack of a significant exposure–response relationship for infections within the therapeutic dose range for daratumumab.
5 Conclusions
Due to a mechanism-based disease–drug exposure interaction, patients with IgG MM may have significantly lower drug concentrations after administration of therapeutic IgG-based mAbs compared with non-IgG myeloma patients. Similar efficacy and safety profiles were observed in IgG and non-IgG patients in response to daratumumab, likely because patients with IgG myeloma may be more responsive to daratumumab while there was no significant exposure–response relationship for safety signals. Careful evaluation of the impact of type of MM and other patient and disease characteristics on drug exposure and clinical outcomes are warranted for all IgG-based mAbs used to treat MM.
References
Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121:482–8.
Santonocito AM, Consoli U, Bagnato S, Milone G, Palumbo GA, Di Raimondo F, et al. Flow cytometric detection of aneuploid CD38(++) plasmacells and CD19(+) B-lymphocytes in bone marrow, peripheral blood and PBSC harvest in multiple myeloma patients. Leuk Res. 2004;28:469–77.
de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8.
Lammerts van Bueren J, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124:3474.
Overdijk MB, Verploegen S, Bogels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7:311–21.
Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol. 2016;197:807–13.
Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128:384–94.
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–66.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis N, Usmani S, et al. Daratumumab, lenalidomide, and dexamethasone for mulitple myeloma. N Engl J Med. 2016;375:1319–31.
McKeage K, Lyseng-Williamson KA. Daratumumab in multiple myeloma: a guide to its use as monotherapy in the EU. Drugs Ther Perspect. 2016;32:463–9.
McKeage K. Daratumumab: first global approval. Drugs. 2016;76:275–81.
DARZALEX™ (daratumumab) injection, for intravenous use [package insert]. Horsham, PA: Janssen Biotech, Inc.; 2016.
Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101–19.
Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–25.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–59.
Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J. Renal function in newly diagnosed multiple myeloma—a demographic study of 1353 patients. The Nordic Myeloma Study Group. Eur J Haematol. 1994;53:207–12.
Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207–19.
Lonial S, Weiss BM, Usmani S, Singhal S, Chari A, Bahlis N, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387:1551–60.
US Food and Drug Administration. Guidance for industry: pharmacokinetics in patients with impaired renal function–study design, data analysis, and impact on dosing and labeling. Available at: http://www.fdagov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm204959pdf. Accessed 28 Feb 2017.
Yan X, Clemens PL, Puchalski T, Lonial S, Lokhorst HM, Orlowski RZ, et al. Target-mediated drug disposition of daratumumab following intravenous infusion in relapsed or refractory multiple myeloma after prior proteasome inhibitors and immunomodulatory drugs: a population pharmacokinetic analysis. Blood. 2015;126:4222.
Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of the therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12:33–43.
Leveque D, Wisniewski S, Jehl F. Pharmacokinetics of therapeutic monoclonal antibodies used in oncology. Anticancer Res. 2005;25:2327–43.
Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–8.
Gibiansky L, Passey C, Roy A, Bello A, Gupta M. Model-based pharmacokinetic analysis of elotuzumab in patients with relapsed/refractory multiple myeloma. J Pharmacokinet Pharmacodyn. 2016;43:243–57.
Xu XS, Yan X, Puchalski T, Lonial S, Lokhorst HM, Voorhees PM, et al. Clinical implications of complex pharmacokinetics for daratumumab dose regimen in patients with relapsed/refractory multiple myeloma. Clin Pharmacol Ther. 2017;101:721–4.
Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128:37–44.
Dosani T, Carlsten M, Maric I, Landgren O. The cellular immune system in myelomagenesis: NK cells and T cells in the development of MM and their uses in immunotherapies. Blood Cancer J. 2015;5:e306.
Casneuf T, Xu XS, Adams H III, Axel A, Verbist B, Liu K, et al. Pharmacodynamic relationship between natural killer cells and daratumumab exposure in relapsed/refractory multiple myeloma. In: Presented at the 21st Congress of the European Hematology Association (EHA); 9–12 June 2016; Copenhagen.
Acknowledgements
The clinical studies were supported by research funding from Janssen Research & Development and Genmab, and the analyses presented here were supported by research funding from Janssen Research & Development. The authors thank the patients who participated in the GEN501 and SIRIUS studies and their families, as well as the study co-investigators, research nurses, and coordinators at each of the clinical sites. Medical writing and editorial support were provided by Erica S. Chevalier-Larsen, PhD, of MedErgy and funded by Janssen Global Services, LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The clinical studies were supported by research funding from Janssen Research & Development and Genmab, and the analyses presented here were supported by Janssen Research & Development.
Conflict of interest
XY, PLC, T. Puchalski, KL, RJ, TA, KL, JJPR, HZ, and XSX are employees of Janssen Research & Development. XY, PLC, T. Puchalski, RJ, TA, KL, JJPR, HZ, and XSX own stock in Johnson & Johnson, and JJRP owns stock in Amgen. SL reports consultancy and research funding from Millennium, Novartis, Bristol-Myers Squibb, Onyx, Celgene, and Janssen. HL reports honoraria from Amgen and honoraria and research funding from Genmab and Janssen. PMV reports consultancy for Janssen, Millennium/Takeda, Celgene, Novartis, Array BioPharma, and Oncopeptides; and research funding from Janssen, Celgene, GlaxoSmithKline, Onyx, and Oncopeptides. SU reports consulting for Celgene, Millennium/Takeda, Onyx, and Sanofi; speaker’s fees from Celgene, Millennium/Takeda, and Onyx; and research funding from Array BioPharma, Celgene, Janssen Oncology, Onyx, Pharmacyclics, and Sanofi. PGR reports being a member of the advisory committee for Genmab. T. Plesner reports research support from Janssen and participation in advisory boards for Janssen, Celgene, AbbVie, and Takeda. RZO reports consulting for Array BioPharma, Bristol-Myers Squibb, Celgene, FORMA Therapeutics, Janssen, Millennium, and Onyx; and research funding from Bristol-Myers Squibb, Celgene, Janssen, Onyx, and Spectrum Pharmaceuticals. NL is an employee of and owns stock in Genmab A/S.
Ethical approval
Study protocols were approved by local ethics committees and conducted per the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The clinical studies were registered at http://www.ClinicalTrials.gov (GEN501, NCT00574288; SIRIUS, NCT01985126).
Informed consent
All patients provided written informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yan, X., Clemens, P.L., Puchalski, T. et al. Influence of Disease and Patient Characteristics on Daratumumab Exposure and Clinical Outcomes in Relapsed or Refractory Multiple Myeloma. Clin Pharmacokinet 57, 529–538 (2018). https://doi.org/10.1007/s40262-017-0598-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0598-1