Abstract
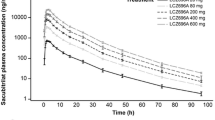
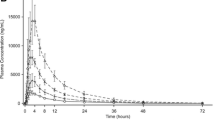
Sacubitril/valsartan (LCZ696) is indicated for the treatment of heart failure with reduced ejection fraction. Absorption of sacubitril/valsartan and conversion of sacubitril (prodrug) to sacubitrilat (neprilysin inhibitor) was rapid with maximum plasma concentrations of sacubitril, sacubitrilat, and valsartan (angiotensin receptor blocker) reaching within 0.5, 1.5–2.0, and 2.0–3.0 h, respectively. With a two-fold increase in dose, an increase in the area under the plasma concentration–time curve was proportional for sacubitril, ~1.9-fold for sacubitrilat, and ~1.7-fold for valsartan in healthy subjects. Following multiple twice-daily administration, steady-state maximum plasma concentration was reached within 3 days, showing no accumulation for sacubitril and valsartan, while ~1.6-fold accumulation for sacubitrilat. Sacubitril is eliminated predominantly as sacubitrilat through the kidney; valsartan is eliminated mainly by biliary route. Drug–drug interactions of sacubitril/valsartan were evaluated with medications commonly used in patients with heart failure including furosemide, warfarin, digoxin, carvedilol, levonorgestrel/ethinyl estradiol combination, amlodipine, omeprazole, hydrochlorothiazide, intravenous nitrates, metformin, statins, and sildenafil. Co-administration with sacubitril/valsartan increased the maximum plasma concentration (~2.0-fold) and area under the plasma concentration–time curve (1.3-fold) of atorvastatin; however, it did not affect the pharmacokinetics of simvastatin. Age, sex, or ethnicity did not affect the pharmacokinetics of sacubitril/valsartan. In patients with heart failure vs. healthy subjects, area under the plasma concentration–time curves of sacubitril, sacubitrilat, and valsartan were higher by approximately 1.6-, 2.1-, and 2.3-fold, respectively. Renal impairment had no significant impact on sacubitril and valsartan area under the plasma concentration–time curves, while the area under the plasma concentration–time curve of sacubitrilat correlated with degree of renal function (1.3-, 2.3-, 2.9-, and 3.3-fold with mild, moderate, and severe renal impairment, and end-stage renal disease, respectively). Moderate hepatic impairment increased the area under the plasma concentration–time curves of valsartan and sacubitrilat ~2.1-fold.




Similar content being viewed by others
Change history
19 May 2017
An erratum to this article has been published.
References
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–52.
Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–8.
MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001–7.
CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9–13.
Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure: randomized aldactone evaluation study investigators. N Engl J Med. 1999;341(10):709–17.
Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
Braunwald E. ACE inhibitors: a cornerstone of the treatment of heart failure. N Engl J Med. 1991;325(5):351–3.
McMurray JJ. CONSENSUS to EMPHASIS: the overwhelming evidence which makes blockade of the renin-angiotensin-aldosterone system the cornerstone of therapy for systolic heart failure. Eur J Heart Fail. 2011;13(9):929–36.
Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Failure. 2014;1(1):4–25.
Volpe M. Natriuretic peptides and cardio-renal disease. Int J Cardiol. 2014;176(3):630–9.
Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50(25):2357–68.
Hawkridge AM, Heublein DM, Bergen HR 3rd, et al. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci USA. 2005;102(48):17442–7.
Niederkofler EE, Kiernan UA, O’Rear J, et al. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circ Heart Fail. 2008;1(4):258–64.
Mangiafico S, Costello-Boerrigter LC, Andersen IA, et al. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34(12):886–893c.
Langenickel TH, Dole WP. Angiotensin receptor-neprilysin inhibition with LCZ696: a novel approach for the treatment of heart failure. Drug Discov Today. 2012;9(4):e131–9.
McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54–61.
Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68(13):1476–88.
Vazir A, Solomon SD. Management strategies for heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10(4):591–8.
Gu J, Noe A, Chandra P, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010;50(4):401–14.
Flarakos J, Du Y, Bedman T, et al. Disposition and metabolism of [C] sacubitril/valsartan (formerly LCZ696) an angiotensin receptor neprilysin inhibitor, in healthy subjects. Xenobiotica. 2016;46(11):986–1000s.
Feng L, Karpinski PH, Sutton P, et al. LCZ696: a dual-acting sodium supramolecular complex. Tetrahedron Lett. 2012;53(3):275–6.
Ayalasomayajula S, Langenickel T, Chandra P, et al. Effect of food on the oral bioavailability of the angiotensin receptor neprilysin inhibitor sacubitril/valsartan (LCZ696) in healthy subjects. Int J Clin Pharmacol Ther. 2016;54(12):1012–8.
Ayalasomayajula S, Jordaan P, Pal P, et al. Assessment of drug interaction potential between LCZ696, an angiotensin receptor neprilysin inhibitor, and digoxin or warfarin. Clin Pharmacol Biopharm. 2015;4:147.
Nadeem S, Asif H, Lakshita C, et al. Pharmacological and pharmaceutical profile of valsartan: a review. J Appl Pharm Sci. 2011;01(04):12–9.
Novartis. Entresto™ (sacubitril and valsartan): US prescribing information. 2015. Available from: http://www.pharma.us.novartis.com. Accessed 21 Nov 2015.
Flesch G, Muller P, Lloyd P. Absolute bioavailability and pharmacokinetics of valsartan, an angiotensin II receptor antagonist, in man. Eur J Clin Pharmacol. 1997;52(2):115–20.
Ayalasomayajula S, Pan W, Han Y, et al. Assessment of drug-drug interaction potential between atorvastatin and LCZ696, a novel angiotensin receptor neprilysin inhibitor, in healthy Chinese male subjects. Eur J Drug Metab Pharmacokinet. 2017;42(2):309–18.
Akahori M, Ayalasomayajula S, Langenickel T, et al. Pharmacokinetics after single ascending dose, food effect, and safety of sacubitril/valsartan (LCZ696), an angiotensin receptor and neprilysin inhibitor, in healthy Japanese subjects. Eur J Drug Metab Pharmacokinet. 2016. [Epub ahead of print].
US Food and Drug Administration. Diovan: prescribing information. 2014. Available from: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/diovan.pdf. Accessed 13 Apr 2017.
Sunkara G, Jiang X, Reynolds C, et al. Effect of food on the oral bioavailability of amlodipine/valsartan and amlodipine/valsartan/hydrochlorothiazide fixed dose combination tablets in healthy subjects. Clin Pharmacol Drug Dev. 2014;3(6):487–92.
Colussi DM, Parisot C, Rossolino ML, et al. Protein binding in plasma of valsartan, a new angiotensin II receptor antagonist. J Clin Pharmacol. 1997;37(3):214–21.
Langenickel TH, Tsubouchi C, Ayalasomayajula S, et al. The effect of LCZ696 (sacubitril/valsartan) on amyloid-beta concentrations in cerebrospinal fluid in healthy subjects. Br J Clin Pharmacol. 2016;81(5):878–90.
Shi J, Wang X, Nguyen J, et al. Sacubitril is selectively activated by carboxylesterase 1 (CES1) in the liver and the activation is affected by CES1 genetic variation. Drug Metab Dispos. 2016;44(4):554–9.
Nakashima A, Kawashita H, Masuda N, et al. Identification of cytochrome P450 forms involved in the 4-hydroxylation of valsartan, a potent and specific angiotensin II receptor antagonist, in human liver microsomes. Xenobiotica. 2005;35(6):589–602.
Waldmeier F, Flesch G, Muller P, et al. Pharmacokinetics, disposition and biotransformation of [14C]-radiolabelled valsartan in healthy male volunteers after a single oral dose. Xenobiotica. 1997;27(1):59–71.
Kobalava Z, Kotovskaya Y, Averkov O, et al. Pharmacodynamic and pharmacokinetic profiles of sacubitril/valsartan (LCZ696) in patients with heart failure and reduced ejection fraction. Cardiovasc Ther. 2016;34(4):191–8.
Myers RP, Cerini R, Sayegh R, et al. Cardiac hepatopathy: clinical, hemodynamic, and histologic characteristics and correlations. Hepatology. 2003;37(2):393–400.
Moller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J. 2013;34(36):2804–11.
Cleland JG, Carubelli V, Castiello T, et al. Renal dysfunction in acute and chronic heart failure: prevalence, incidence and prognosis. Heart Fail Rev. 2012;17(2):133–49.
Prasad PP, Yeh CM, Gurrieri P, et al. Pharmacokinetics of multiple doses of valsartan in patients with heart failure. J Cardiovasc Pharmacol. 2002;40(5):801–7.
Prasad P, Kalbag J, Hester A. Assessment of dose proportionality of an angiotensin II receptor blocker, valsartan, following single doses of 80, 160 and 320 mg to healthy subjects (abstract). Pharm Sci. 1998;S144.
Ayalasomayajula SP, Langenickel TH, Jordaan P, et al. Effect of renal function on the pharmacokinetics of LCZ696 (sacubitril/valsartan), an angiotensin receptor neprilysin inhibitor. Eur J Clin Pharmacol. 2016;72:1065–73.
Kulmatycki K, Langenickel T, Ng D, et al. Pharmacokinetics of single-dose LCZ696 in subjects with mild and moderate hepatic impairment. Clin Pharmacol Drug Dev. 2014;3(Suppl. 1):21.
Writing Committee Members. ACC/AHA Task Force members. ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Card Fail. 2016;22(9):659–69.
Ayalasomayajula S, Langenikel T, Malcolm K, et al. In vitro and clinical evaluation of OATP-mediated drug interaction potential of sacubitril/valsartan (LCZ696). J Clin Pharm Ther. 2016;41:424–31.
Hsiao HL, Langenickel TH, Greeley M, et al. Pharmacokinetic drug–drug interaction assessment between LCZ696, an angiotensin receptor neprilysin inhibitor, and hydrochlorothiazide, amlodipine, or carvedilol. Clin Pharmacol Drug Dev. 2015;4(6):407–17.
Gan L, Jiang X, Mendonza A, et al. Pharmacokinetic drug-drug interaction assessment of LCZ696 (an angiotensin receptor neprilysin inhibitor) with omeprazole, metformin or levonorgestrel-ethinyl estradiol in healthy subjects. Clin Pharmacol Drug Develop. 2015;5:27–39.
Tenero D, Boike S, Boyle D, et al. Steady-state pharmacokinetics of carvedilol and its enantiomers in patients with congestive heart failure. J Clin Pharmacol. 2000;40(8):844–53.
Oldham HG, Clarke SE. In vitro identification of the human cytochrome P450 enzymes involved in the metabolism of R(+)- and S(−)-carvedilol. Drug Metab Dispos. 1997;25(8):970–7.
Bachmakov I, Werner U, Endress B, et al. Characterization of beta-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam Clin Pharmacol. 2006;20(3):273–82.
Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194–9.
Budzynski J, Pulkowski G, Suppan K, et al. Improvement in health-related quality of life after therapy with omeprazole in patients with coronary artery disease and recurrent angina-like chest pain: a double-blind, placebo-controlled trial of the SF-36 survey. Health Qual Life Outcomes. 2011;9:77.
Ogawa R, Echizen H. Drug-drug interaction profiles of proton pump inhibitors. Clin Pharmacokinet. 2010;49(8):509–33.
Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol. 2008;64(10):935–51.
Saydam M, Takka S. Bioavailability file: valsartan. FABAD J Pharm Sci. 2007;32:185–96.
Hirsh J. Oral anticoagulant drugs. N Engl J Med. 1991;324(26):1865–75.
Black DJ, Kunze KL, Wienkers LC, et al. Warfarin-fluconazole. II. A metabolically based drug interaction: in vivo studies. Drug Metab Dispos. 1996;24(4):422–8.
Wittkowsky AK. Warfarin and other coumarin derivatives: pharmacokinetics, pharmacodynamics, and drug interactions. Semin Vasc Med. 2003;3(3):221–30.
Serlin MJ, Breckenridge AM. Drug interactions with warfarin. Drugs. 1983;25(6):610–20.
National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III): final report. Circulation. 2002;106(25):3143–421.
European Association for Cardiovascular Prevention and Rehabilitation, Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–818.
McMurray JJ, Packer M, Desai AS, et al. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Eur J Heart Fail. 2014;16(7):817–25.
Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158(3):693–705.
Niemi M. Role of OATP transporters in the disposition of drugs. Pharmacogenomics. 2007;8(7):787–802.
Kindla J, Fromm MF, Konig J. In vitro evidence for the role of OATP and OCT uptake transporters in drug-drug interactions. Expert Opin Drug Metab Toxicol. 2009;5(5):489–500.
Higgins JW, Bao JQ, Ke AB, et al. Utility of Oatp1a/1b-knockout and OATP1B1/3-humanized mice in the study of OATP-mediated pharmacokinetics and tissue distribution: case studies with pravastatin, atorvastatin, simvastatin, and carboxydichlorofluorescein. Drug Metab Dispos. 2014;42(1):182–92.
Kunze A, Huwyler J, Camenisch G, et al. Prediction of organic anion-transporting polypeptide 1B1- and 1B3-mediated hepatic uptake of statins based on transporter protein expression and activity data. Drug Metab Dispos. 2014;42(9):1514–21.
Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80(6):565–81.
Vallon V, Rieg T, Ahn SY, et al. Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol. 2008;294(4):F867–73.
Yamashiro W, Maeda K, Hirouchi M, et al. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug Metab Dispos. 2006;34(7):1247–54.
Ponto LL, Schoenwald RD. Furosemide (frusemide): a pharmacokinetic/pharmacodynamic review (Part I). Clin Pharmacokinet. 1990;18(5):381–408.
Bindschedler M, Degen P, Flesch G, et al. Pharmacokinetic and pharmacodynamic interaction of single oral doses of valsartan and furosemide. Eur J Clin Pharmacol. 1997;52(5):371–8.
Eichhorn EJ, Gheorghiade M. Digoxin. Prog Cardiovasc Dis. 2002;44(4):251–66.
Gheorghiade M, van Veldhuisen DJ, Colucci WS. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation. 2006;113(21):2556–64.
de Lannoy IA, Silverman M. The MDR1 gene product, P-glycoprotein, mediates the transport of the cardiac glycoside, digoxin. Biochem Biophys Res Commun. 1992;189(1):551–7.
Hori R, Okamura N, Aiba T, et al. Role of P-glycoprotein in renal tubular secretion of digoxin in the isolated perfused rat kidney. J Pharmacol Exp Ther. 1993;266(3):1620–5.
Wessler JD, Grip LT, Mendell J, et al. The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol. 2013;61(25):2495–502.
Hill NS, Antman EM, Green LH, et al. Intravenous nitroglycerin: a review of pharmacology, indications, therapeutic effects and complications. Chest. 1981;79(1):69–76.
Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53(Suppl. 1):5S–12S.
Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–357.
Niemeyer C, Hasenfuss G, Wais U, et al. Pharmacokinetics of hydrochlorothiazide in relation to renal function. Eur J Clin Pharmacol. 1983;24(5):661–5.
Beermann B, Groschinsky-Grind M, Rosen A. Absorption, metabolism, and excretion of hydrochlorothiazide. Clin Pharmacol Ther. 1976;19(5 Pt 1):531–7.
Chung N, Baek S, Chen MF, et al. Expert recommendations on the challenges of hypertension in Asia. Int J Clin Pract. 2008;62(9):1306–12.
Wang JG, Kario K, Lau T, et al. Use of dihydropyridine calcium channel blockers in the management of hypertension in Eastern Asians: a scientific statement from the Asian Pacific Heart Association. Hypertens Res. 2011;34(4):423–30.
Wang JG, Li Y. Characteristics of hypertension in Chinese and their relevance for the choice of antihypertensive drugs. Diabetes Metab Res. 2012;28:67–72.
Ogihara T, Kikuchi K, Matsuoka H, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2009). Hypertens Res. 2009;32(1):3–107.
Zhu Y, Wang F, Li Q, et al. Amlodipine metabolism in human liver microsomes and roles of CYP3A4/5 in the dihydropyridine dehydrogenation. Drug Metab Dispos. 2014;42(2):245–9.
Zhang H, Cui D, Wang B, et al. Pharmacokinetic drug interactions involving 17alpha-ethinylestradiol: a new look at an old drug. Clin Pharmacokinet. 2007;46(2):133–57.
Moreno I, Quinones L, Catalan J, et al. Influence of CYP3A4/5 polymorphisms in the pharmacokinetics of levonorgestrel: a pilot study [in Spanish]. Biomedica. 2012;32(4):570–7.
Rakugi H, Kario K, Yamaguchi M, et al. Efficacy and safety of LCZ696 compared with olmesartan in Japanese patients with systolic hypertension. Hypertens. 2014;64:A474.
Ito S, Satoh M, Tamaki Y, et al. Safety and efficacy of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Japanese patients with hypertension and renal dysfunction. Hypertens Res. 2015;38(4):269–75.
Gan L, Langenickel T, Petruck J, et al. Effects of age and sex on the pharmacokinetics of LCZ696, an angiotensin receptor neprilysin inhibitor. J Clin Pharmacol. 2016;56(1):78–86.
Jhund PS, Fu M, Bayram E, et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015;36(38):2576–84.
Marsh S, Xiao M, Yu J, et al. Pharmacogenomic assessment of carboxylesterases 1 and 2. Genomics. 2004;84(4):661–8.
Suzaki Y, Uemura N, Takada M, et al. The effect of carboxylesterase 1 (CES1) polymorphisms on the pharmacokinetics of oseltamivir in humans. Eur J Clin Pharmacol. 2013;69(1):21–30.
Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70.
Brookman LJ, Rolan PE, Benjamin IS, et al. Pharmacokinetics of valsartan in patients with liver disease. Clin Pharmacol Ther. 1997;62(3):272–8.
Acknowledgements
The authors acknowledge Iain O’Neill (contracted to Global Medical and Clinical Services, Novartis Ireland Ltd.) for providing editorial support.
Author information
Authors and Affiliations
Contributions
All authors were involved in the preparation of the manuscript, provided input, and reviewed the final draft for the publication.
Corresponding author
Ethics declarations
Conflict of interest
All authors are employees of Novartis except Surya Ayalasomayajula, who was an employee of Novartis during the development of the manuscript.
Funding
All clinical pharmacokinetic studies are sponsored by Novartis Pharmaceuticals Co.
Additional information
An erratum to this article is available at https://doi.org/10.1007/s40262-017-0558-9.
Rights and permissions
About this article
Cite this article
Ayalasomayajula, S., Langenickel, T., Pal, P. et al. Clinical Pharmacokinetics of Sacubitril/Valsartan (LCZ696): A Novel Angiotensin Receptor-Neprilysin Inhibitor. Clin Pharmacokinet 56, 1461–1478 (2017). https://doi.org/10.1007/s40262-017-0543-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0543-3