Abstract
Introduction
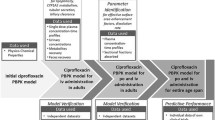
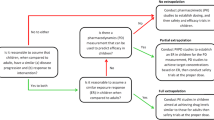
Modeling and simulation approaches are increasingly being utilized in pediatric drug development. Physiologically based pharmacokinetic (PBPK) modeling offers an enhanced ability to predict age-related changes in pharmacokinetics in the pediatric population.
Methods
In the current study, adult PBPK models were developed for the renally excreted drugs linezolid and emtricitabine. PBPK models were then utilized to predict pharmacokinetics in pediatric patients for various age groups from the oldest to the youngest patients in a stepwise approach.
Results
Pharmacokinetic predictions for these two drugs in the pediatric population, including infants and neonates, were within a twofold range of clinical observations. Based on this study, linezolid and emtricitabine pediatric PBPK models incorporating the ontogeny in renal maturation describe the pharmacokinetic differences between adult and pediatric populations, even though the contribution of renal clearance to the total clearance of two drugs was very different (30 % for linezolid vs. 86 % for emtricitabine).
Conclusion
These results suggest that PBPK modeling may provide one option to help predict the pharmacokinetics of renally excreted drugs in neonates and infants.







Similar content being viewed by others
References
FDA. Food and Drug Administration Safety and Innovation Act (FDASIA). Silver Spring: FDA; 2012.
Wang J, Avant D, Green D, Seo S, Fisher J, Mulberg AE, et al. A survey of neonatal pharmacokinetic and pharmacodynamic studies in pediatric drug development. Clin Pharmacol Ther. 2015;98(3):328–35.
van den Anker J, Allegaert K. Clinical pharmacology in neonates and young infants: the benefit of a population-tailored approach. Expert Rev Clin Pharmacol. 2012;5(1):5–8.
Laughon MM, Avant D, Tripathi N, Hornik CP, Cohen-Wolkowiez M, Clark RH, et al. Drug labeling and exposure in neonates. JAMA Pediatr. 2014;168(2):130–6.
Johnson TN. The problems in scaling adult drug doses to children. Arch Dis Child. 2008;93(3):207–11.
Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacomet Syst Pharmacol. 2014;3:e150.
Advisory Committee for Pharmaceutical Science and Clinical Pharmacology. Clinical pharmacology aspects of pediatric clinical trial design and dosing to optimize pediatric drug development. Silver Spring: FDA; 2012.
Wang J, Edginton AN, Avant D, Burckart GJ. Predicting neonatal pharmacokinetics from prior data using population pharmacokinetic modeling. J Clin Pharmacol. 2015;55(10):1175–83.
Johnson TN, Zhou D, Bui KH. Development of physiologically based pharmacokinetic model to evaluate the relative systemic exposure to quetiapine after administration of IR and XR formulations to adults, children and adolescents. Biopharm Drug Dispos. 2014;35(6):341–52.
Khalil F, Laer S. Physiologically based pharmacokinetic models in the prediction of oral drug exposure over the entire pediatric age range-sotalol as a model drug. AAPS J. 2014;16(2):226–39.
Jiang XL, Zhao P, Barrett JS, Lesko LJ, Schmidt S. Application of physiologically based pharmacokinetic modeling to predict acetaminophen metabolism and pharmacokinetics in children. CPT Pharmacomet Syst Pharmacol. 2013;2:e80.
Emoto C, Fukuda T, Johnson TN, Adams DM, Vinks AA. Development of a pediatric physiologically based pharmacokinetic model for sirolimus: applying principles of growth and maturation in neonates and infants. CPT Pharmacomet Syst Pharmacol. 2015;4(2):e17.
Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24(1):67–76.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45(9):931–56.
US Food and Drug Administration. Guidance for Industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. In: Center for Drug Evaluation and Research (CDER), editor. Silver Spring: US FDA; 2010. http://www.fda.gov/downloads/Drugs/…/Guidances/UCM204959.pdf. Accessed 12 Aug 2016.
Wynalda MA, Hauer MJ, Wienkers LC. Oxidation of the novel oxazolidinone antibiotic linezolid in human liver microsomes. Drug Metab Dispos. 2000;28(9):1014–7.
Nakatani-Freshwater T, Taft DR. Renal excretion of emtricitabine I: effects of organic anion, organic cation, and nucleoside transport inhibitors on emtricitabine excretion. J Pharm Sci. 2008;97(12):5401–10.
Gutierrez F, Fulladosa X, Barril G, Domingo P. Renal tubular transporter-mediated interactions of HIV drugs: implications for patient management. AIDS Rev. 2014;16(4):199–212.
Molina JM, Cox SL. Emtricitabine: a novel nucleoside reverse transcriptase inhibitor. Drugs Today (Barc). 2005;41(4):241–52.
Stalker DJ, Jungbluth GL. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet. 2003;42(13):1129–40.
Leong R, Vieira ML, Zhao P, Mulugeta Y, Lee CS, Huang SM, et al. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin Pharmacol Ther. 2012;91(5):926–31.
Barrett JS, Della Casa Alberighi O, Laer S, Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther. 2012;92(1):40–9.
Maharaj AR, Barrett JS, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013;15(2):455–64.
Drugs@fda. Clinical pharmacology and biopharmaceutics review for NDA 021130. FDA; 2000. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21130_Zyvox_biopharmr.pdf.
Drugs@fda. Clinical pharmacology and biopharmaceutics review on NDA 021896. FDA; 2005. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021896s000_ClinPharmR.pdf.
Gandelman K, Zhu T, Fahmi OA, Glue P, Lian K, Obach RS, et al. Unexpected effect of rifampin on the pharmacokinetics of linezolid: in silico and in vitro approaches to explain its mechanism. J Clin Pharmacol. 2011;51(2):229–36.
Brier ME, Stalker DJ, Aronoff GR, Batts DH, Ryan KK, O’Grady M, et al. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob Agents Chemother. 2003;47(9):2775–80.
Xia B, Heimbach T, Gollen R, Nanavati C, He H. A simplified PBPK modeling approach for prediction of pharmacokinetics of four primarily renally excreted and CYP3A metabolized compounds during pregnancy. AAPS J. 2013;15(4):1012–24.
Rodgers T, Leahy D, Rowland M. Tissue distribution of basic drugs: accounting for enantiomeric, compound and regional differences amongst beta-blocking drugs in rat. J Pharm Sci. 2005;94(6):1237–48.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Rodgers T, Rowland M. Mechanistic approaches to volume of distribution predictions: understanding the processes. Pharm Res. 2007;24(5):918–33.
Vieira ML, Zhao P, Berglund EG, Reynolds KS, Zhang L, Lesko LJ, et al. Predicting drug interaction potential with a physiologically based pharmacokinetic model: a case study of telithromycin, a time-dependent CYP3A inhibitor. Clin Pharmacol Ther. 2012;91(4):700–8.
Pierrat A, Gravier E, Saunders C, Caira MV, Ait-Djafer Z, Legras B, et al. Predicting GFR in children and adults: a comparison of the Cockcroft–Gault, Schwartz, and modification of diet in renal disease formulas. Kidney Int. 2003;64(4):1425–36.
T’Jollyn H, Snoeys J, Vermeulen A, Michelet R, Cuyckens F, Mannens G, et al. Physiologically based pharmacokinetic predictions of tramadol exposure throughout pediatric life: an analysis of the different clearance contributors with emphasis on CYP2D6 maturation. AAPS J. 2015;17(6):1376–87.
Claassen K, Thelen K, Coboeken K, Gaub T, Lippert J, Allegaert K, et al. Development of a physiologically-based pharmacokinetic model for preterm neonates: evaluation with in vivo data. Curr Pharm Des. 2015;21(39):5688–98.
Abduljalil K, Jamei M, Rostami-Hodjegan A, Johnson TN. Changes in individual drug-independent system parameters during virtual paediatric pharmacokinetic trials: introducing time-varying physiology into a paediatric PBPK model. AAPS J. 2014;16(3):568–76.
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos. 2015;43(11):1823–37.
Fieller EC. Some problems in interval estimation. J R Stat Soc. 1954;16(2):175–85.
Agras PI, Tarcan A, Baskin E, Cengiz N, Gurakan B, Saatci U. Acute renal failure in the neonatal period. Ren Fail. 2004;26(3):305–9.
Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol. 2009;24(2):265–74.
Yu G, Zheng QS, Li GF. Similarities and differences in gastrointestinal physiology between neonates and adults: a physiologically based pharmacokinetic modeling perspective. AAPS J. 2014;16(6):1162–6.
Acknowledgments
We thank Dr. Gerri Baer, Office of Pediatric Therapeutics, US FDA, for her critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used for this work or the preparation of the manuscript.
Disclosure
Dr Kenta Yoshida was supported in part by an appointment to the Research Participation Program at the Center for Drug Evaluation and Research, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the US Food and Drug Administration (FDA).
Conflict of interest
Peng Duan, Jeffery W. Fisher, Kenta Yoshida, Lei Zhang, Gilbert J. Burckart, and Jian Wang have no conflicts of interest that are directly relevant to the content of this manuscript.
Disclaimer
The contents of this manuscript reflect the views of the authors and should not be interpreted as representing the US FDA’s views or policies. No official support or endorsement by the FDA is intended or should be inferred. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the FDA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, P., Fisher, J.W., Yoshida, K. et al. Physiologically Based Pharmacokinetic Prediction of Linezolid and Emtricitabine in Neonates and Infants. Clin Pharmacokinet 56, 383–394 (2017). https://doi.org/10.1007/s40262-016-0445-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0445-9