Abstract
Objectives
The aim of this study was to develop a population pharmacokinetic (PK)/pharmacodynamic (PD) model for describing plasma lusutrombopag concentrations and platelet response following oral lusutrombopag dosing and for evaluating covariates in the PK/PD profiles.
Methods
A population PK/PD model was developed using a total of 2539 plasma lusutrombopag concentration data and 1408 platelet count data from 78 healthy adult subjects following oral single and multiple (14-day once-daily) dosing. Covariates in PK and PK/PD models were explored for subject age, body weight, sex, and ethnicity.
Results
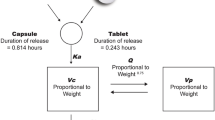
A three-compartment model with first-order rate and lag time for absorption was selected as a PK model. A three-transit and one-platelet compartment model with a sigmoid E max model for drug effect and feedback of platelet production was selected as the PD model. The PK and PK/PD models well described the plasma lusutrombopag concentrations and the platelet response, respectively. Body weight was a significant covariate in PK. The bioavailability of non-Japanese subjects (White and Black/African American subjects) was 13 % lower than that of Japanese subjects, while the simulated platelet response profiles using the PK/PD model were similar between Japanese and non-Japanese subjects. There were no significant covariates of the tested background data including age, sex, and ethnicity (Japanese or non-Japanese) for the PD sensitivity.
Conclusion
A population PK/PD model was developed for lusutrombopag and shown to provide good prediction for the PK/PD profiles. The model could be used as a basic PK/PD model in the drug development of lusutrombopag.





Similar content being viewed by others
References
Szilvassy SJ. Haematopoietic stem and progenitor cell-targeted therapies for thrombocytopenia. Expert Opin Biol Ther. 2006;6:983–92.
Izumi N, Tateishi R, Seike M, Kudo M, Tamai H, Kawazoe S, Tanaka K, Osaki Y, Yamamoto K, Imawari M. Once-daily oral lusutrombopag, alternative to platelet transfusion in thrombocytopenic patients with chronic liver disease undergoing radiofrequency ablation: results from a phase 2b, randomized, double-blind study. In: The International Liver Congress™ 2014, 49th Annual Meeting of the European Association for the Study of the Liver, London, UK; April 9-13 2014.
Sheiner LB, Steimer JL. Pharmacokinetic/pharmacodynamic modeling in drug development. Annu Rev Pharmacol Toxicol. 2000;40:67–95.
Chien JY, Friedrich S, Heathman MA, de Alwis DP, Sinha V. Pharmacokinetics/pharmacodynamics and the stages of drug development: role of modeling and simulation. AAPS J. 2005;7:E544–59.
Samtani MN, Perez-Ruixo JJ, Brown KH, Cerneus D, Molloy CJ. Pharmacokinetic and pharmacodynamic modeling of pegylated thrombopoietin mimetic peptide (PEG-TPOm) after single intravenous dose administration in healthy subjects. J Clin Pharmacol. 2009;49(3):336–50.
Gibiansky E, Zhang J, Williams D, Wang Z, Ouellet D. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol. 2011;51:842–56.
Hayes S, Ouellet D, Zhang J, Wire MB, Gibiansky E. Population PK/PD modeling of eltrombopag in healthy volunteers and patients with immune thrombocytopenic purpura and optimization of response-guided dosing. J Clin Pharmacol. 2011;51:1403–17.
Kaushansky K. Thrombopoetin. N Engl J Med. 1998;339:746–54.
Syrjälä MT, Savolainen S, Nieminen U, Gripenberg J, Liewendahl K, Ikkala E. Splenic dynamics of indium-111 labeled platelets in idiopathic thrombocytopenic purpura. J Nucl Med. 1989;30:1546–9.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Beal SL, Sheiner LB, Boeckmann AJ. NONMEM users guide, 1989–2006. Ellicott City (MD): Icon Development Solutions.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit: a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57.
Krzyzanski W, Ramakrishnan R, Jusko WJ. Basic pharmacodynamic models for agents that alter production of natural cells. J Pharmacokinet Biopharm. 1999;27(5):467–89.
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20(24):4713–21.
Wolber EM, Jelkmann W. Thrombopoietin: the novel hepatic hormone. News Physiol Sci. 2002;17:6–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors, Takayuki Katsube, Toru Ishibashi, Takeshi Kano, and Toshihiro Wajima, are all employees of Shionogi & Co., Ltd.
Funding
This study was supported by Shionogi & Co., Ltd.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40262_2016_411_MOESM2_ESM.tiff
Supplementary material 2 (TIFF 12656 kb) Supplemental Fig. S1 Relationships of empirical Bayes-estimated CL/F or V2/F of the base model and background data. Open circles: parameters of Japanese subjects. Closed circles: parameters for non-Japanese subjects. Solid line: a LOESS smoother line. Box plot: a thick center line represents the median, top, and base of the box represent the first and third quartiles [interquartile range (IQR)], whiskers represent the most extreme data within 1.5 × IQR, and circles represent outliers beyond 1.5 × IQR. CL/F apparent total clearance, V2/F apparent central volume, Jpn Japanese, Non Jpn non-Japanese
40262_2016_411_MOESM3_ESM.tiff
Supplementary material 3 (TIFF 21357 kb) Supplemental Fig. S2 Goodness-of-fit plots for the final PK model. Solid line: y = x in upper figures and y = 0 in lower figures. Dotted line: a LOESS smoother line. DV observed data, CWRES conditional weighted residuals, IPRED individual-predicted data, PK/PD pharmacokinetic/pharmacodynamic, PRED population-predicted data
40262_2016_411_MOESM4_ESM.tiff
Supplementary material 4 (TIFF 6328 kb) Supplemental Fig. S3 Relationships of empirical Bayes-estimated EC50 of the base model and background data. Open circles: parameters of Japanese subjects. Closed circles: parameters for non-Japanese subjects. Solid line: a LOESS smoother line. Box plot: a thick center line represents the median, top, and base of the box represent the first and third quartiles [interquartile range (IQR)], whiskers represent the most extreme data within 1.5 × IQR, and circles represent outliers beyond 1.5 × IQR. EC 50 plasma S-888711 concentration achieving 50 % of Emax, Jpn Japanese, Non Jpn non-Japanese
40262_2016_411_MOESM5_ESM.tiff
Supplementary material 5 (TIFF 21357 kb) Supplemental Fig. S4 Goodness-of-fit plots for the final PK/PD model. Solid line: y = x in upper figures and y = 0 in lower figures. Dotted line: a LOESS smoother line. DV observed data, CWRES conditional weighted residuals, IPRED individual-predicted data, PK/PD pharmacokinetic/pharmacodynamic, PRED population-predicted data
Rights and permissions
About this article
Cite this article
Katsube, T., Ishibashi, T., Kano, T. et al. Population Pharmacokinetic and Pharmacodynamic Modeling of Lusutrombopag, a Newly Developed Oral Thrombopoietin Receptor Agonist, in Healthy Subjects. Clin Pharmacokinet 55, 1423–1433 (2016). https://doi.org/10.1007/s40262-016-0411-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0411-6