Abstract
Background and Objectives
We propose a strategy for studying ethnopharmacology by conducting sequential physiologically based pharmacokinetic (PBPK) prediction (a ‘bottom-up’ approach) and population pharmacokinetic (popPK) confirmation (a ‘top-down’ approach), or in reverse order, depending on whether the purpose is ethnic effect assessment for a new molecular entity under development or a tool for ethnic sensitivity prediction for a given pathway. The strategy is exemplified with bitopertin.
Methods
A PBPK model was built using Simcyp® to simulate the pharmacokinetics of bitopertin and to predict the ethnic sensitivity in clearance, given pharmacokinetic data in just one ethnicity. Subsequently, a popPK model was built using NONMEM® to assess the effect of ethnicity on clearance, using human data from multiple ethnic groups. A comparison was made to confirm the PBPK-based ethnic sensitivity prediction, using the results of the popPK analysis.
Results
PBPK modelling predicted that the bitopertin geometric mean clearance values after 20 mg oral administration in Caucasians would be 1.32-fold and 1.27-fold higher than the values in Chinese and Japanese, respectively. The ratios of typical clearance in Caucasians to the values in Chinese and Japanese estimated by popPK analysis were 1.20 and 1.17, respectively. The popPK analysis results were similar to the PBPK modelling results.
Conclusion
As a general framework, we propose that PBPK modelling should be considered to predict ethnic sensitivity of pharmacokinetics prior to any human data and/or with data in only one ethnicity. In some cases, this will be sufficient to guide initial dose selection in different ethnicities. After clinical trials in different ethnicities, popPK analysis can be used to confirm ethnic differences and to support dose justification and labelling. PBPK modelling prediction and popPK analysis confirmation can complement each other to assess ethnic differences in pharmacokinetics at different drug development stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Physiologically based pharmacokinetic (PBPK) prediction and population pharmacokinetic confirmation can complement each other to assess ethnic differences in the pharmacokinetics of new molecular entities (NMEs) at different drug development stages. |
For NMEs involving well-defined pharmacokinetic processes with well-established PBPK models, especially for those low-ethnic-sensitivity pathways that are defined, dedicated pharmacokinetic bridging studies may not be needed. |
Successful application of this strategy may be facilitated by an academic–industry–regulatory consortium to collect ethnic-specific system data and to develop and validate PBPK models of the major pharmacokinetic processes. |
1 Introduction
In recent years, there has been a dramatic increase in the number of global clinical trials [1, 2]. This trend offers opportunities for cost saving and recruitment acceleration, as well as minimizing duplication of clinical data and shortening the drug approval gap among regions. However, heterogeneity due to ethnic differences is a potential concern and needs to be addressed to allow successful global clinical trials [3]. Traditional exploratory pharmacokinetic/pharmacodynamic (PK/PD) bridging studies that assess ethnic differences face a number of challenges. Financially, it is costly to frontload early exploratory PK/PD bridging studies for every molecule entering phase 1 and, technically, such studies may not be able to draw adequate conclusions about the size of the ethnic effect, because of large PK/PD variability, small sample sizes or sex imbalances between ethnic groups. Ethnic sensitivity prediction using physiologically based pharmacokinetic (PBPK) modelling (a ‘bottom-up’ approach) offers the potential to support early decision making on the timing and design of bridging studies because it can be performed prior to first-in-man studies and/or at a time when pharmacokinetic data in only one ethnicity are available. Then, when in vivo pharmacokinetic data in different ethnic groups are available, population pharmacokinetic (popPK) analysis (a ‘top-down’ approach) can be used to confirm the PBPK prediction and fulfil regulatory requirements.
Bitopertin (RG1678, RO4917838) is a glycine reuptake inhibitor, which is postulated to improve N-methyl-d-aspartate (NMDA) receptor hypofunction by increasing the synaptic concentration of glycine, an obligatory co-agonist at the NMDA receptor [4–6]. Bitopertin is a lipophilic molecule showing rapid and extensive distribution into tissues, and slow clearance in Caucasians [7]. Absorption of bitopertin is good (simulated bioavailability by a validated PBPK model in humans is >90 % at doses <80 mg) but slightly less than dose proportional at doses >50 mg because of the low solubility and incomplete absorption of the dose [8]. In vitro and in vivo studies have shown that cytochrome P450 (CYP) 3A4 is the major enzyme responsible for metabolism of bitopertin [7].
We propose a strategy for ethnopharmacology whereby bottom-up and top-down approaches are applied in a complementary fashion. For a new molecular entity (NME), PBPK modelling can be applied to make an early prediction of ethnic sensitivity. As clinical data sets become available, the effects of ethnic differences are quantified through a robust popPK analysis. This strategy is exemplified with bitopertin. A systematic knowledge of ethnic variations in physiology, enzymes and transporters is required for successful application of this strategy.
2 Methods
2.1 General
PBPK simulations were performed using the Simcyp® Population-Based Simulator version 14 (Simcyp Ltd, Sheffield, UK). PopPK analysis was conducted using NONMEM® version 7.2 (Icon Development Solutions, Hanover, MD, USA).
2.2 Clinical Data
The study included 101 healthy adult subjects (61 Caucasians, 16 Chinese and 24 Japanese) from four clinical pharmacokinetic studies. Two studies were conducted in Caucasian subjects, one study in Chinese subjects and one study in Japanese subjects. In these studies, a single dose of bitopertin, ranging from 3 to 240 mg, was administered orally to fasting individuals. The study details are summarized in Table 1. All studies were approved by independent ethics committees, and all study participants provided written informed consent before enrolment.
2.3 PBPK Model
The original bitopertin PBPK model was built pre-clinically using GastroPlus™ version 8.0 (Simulations Plus, Inc., Lancaster, CA, USA) and was used for the first prediction of human pharmacokinetics as described by Parrott et al. [7]. For use in ethnic sensitivity simulations, we transferred the model to Simcyp®, where ethnic populations are available for Caucasian, Chinese and Japanese. The input parameters for the bitopertin model are summarized in Electronic Supplementary Material Table 1. Since the focus of this study was to assess ethnic differences in oral clearance (CL/F), the GastroPlus™ absorption model was replaced by a simple first-order absorption model using the absorbed fraction of the dose (F a), based on the dose-dependent values reported [7]. The absorption rate constant (K a) was the geometric mean (GM) value estimated on the basis of the concentration data in Caucasians, using Phoenix software (WinNonlin models version 6.4, Pharsight Corp., Cary, NC, USA). The variability in K a was large (coefficient of variation [CV] 73.3 %). The same K a was used at different dose levels for PBPK simulations in the three ethnicities. The demographic databases of Caucasians, Chinese and Japanese were as provided in Simcyp®. F a at the same dose level, the intrinsic catalytic activity of CYP3A4 per unit amount of enzyme variant and tissue composition was assumed to be the same in Caucasians, Chinese and Japanese. The mean abundances of CYP3A4 in the liver in Caucasians, Chinese and Japanese were 137, 120 and 122 pmol/mg, respectively [9, 10]. The liver weight was estimated on the basis of the body surface area (BSA) [liver weight = 0.722 × BSA1.176] with an additional scalar of 0.85 in Chinese, based on literature indicating that Chinese have significantly smaller livers than Caucasians and Japanese [9]. Within Simcyp®, we set the age range and proportion of female subjects for the selected ethnic populations and then allowed Simcyp® to use its built-in algorithms and equations to generate virtual populations similar to the actual study populations. The details of how Simcyp® generates a population have been described by Howgate et al. [11]. The simulations followed the reported study designs and conditions as closely as possible, including the numbers of subjects, pharmacokinetic sampling duration, route of administration, dose and fasted/fed status. Ten trial simulations were performed for each study.
After the bitopertin PBPK model was evaluated, CL/F of 20 mg of bitopertin in the age range of 20–70 years, with 50 % female subjects, was simulated in Caucasians (n = 1000), Chinese (n = 1000) and Japanese (n = 1000), and was used to assess ethnic differences.
2.4 PopPK Model
Population pharmacokinetic analysis was performed using nonlinear mixed-effects modelling with first-order conditional estimation with interaction (FOCEI). Models were statistically compared using a likelihood ratio test (LRT) on the difference in the objective function value (OFV). Statistical significance was set at p < 0.01 (ΔOFV >6.63). The slightly less than dose-proportional increase in the area under the plasma concentration–time curve (AUC) [non-compartmental analysis] over the dose range of 3–240 mg in Caucasians was assumed to be caused by a nonlinear F a rather than nonlinear elimination [7]. Therefore, the effect of the dose on F a was described using a nonlinear model, and F a relative to that of the lowest dose at different dose levels was estimated. To describe the nonlinearity in the maximum plasma drug concentration (C max) and AUC at different magnitudes, a saturable Michaelis–Menten absorption model was used. Exponential errors following a log-normal distribution were used to describe the between-subject variability (BSV) in the pharmacokinetic parameters. A combined proportional and additive error model was used to describe the residual unexplained variability (RUV). The effects of demographic factors (age, weight, BSA, height, body mass index [BMI], sex and ethnicity) on the model parameters were investigated to explain the variability. A stepwise forward inclusion procedure (p < 0.01; decrease in OFV >6.63) was performed to build the full model, and a stepwise backward elimination procedure (p < 0.001; increase in OFV >10.83) was applied to determine the final model. The final model was evaluated using a visual predictive check (VPC): a total of 1000 data sets were simulated using the parameter estimates in the final model.
2.5 Statistical Analysis
Population pharmacokinetic parameters are generally considered to be log normally distributed. Hence, the values of the geometric means and 95 % confidence intervals (CIs) were used to compare CL/F.
CL/F was calculated from a non-compartmental analysis for the observed and PBPK-simulated concentration–time data, using Phoenix software. The figures were drawn using R software version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Subject Characteristics
The demographics of the Caucasian, Chinese and Japanese subjects in the in vivo studies can be seen in Table 2.
3.2 PBPK Model Evaluation
The geometric mean CL/F predicted by the PBPK model in the Caucasian, Chinese and Japanese populations after administration of different dose levels of bitopertin can be seen in Table 3. All ratios of predicted to observed geometric mean CL/F values were within 2-fold in the three populations. Most (13/16) of the ratios were within 0.8–1.25 in Caucasians, except at the 6, 20 and 240 mg dose levels. All of the ratios were within 0.8–1.25 in the Chinese and Japanese populations.
These simulations demonstrated that bitopertin PBPK models were able to predict CL/F of bitopertin well.
3.3 Prediction of Ethnic Sensitivity in Clearance, Using PBPK Modelling
The demographics of the 1000 simulated Caucasian, Chinese and Japanese subjects can be seen in Electronic Supplementary Material Table 2. The predicted geometric mean CL/F values in Caucasian, Chinese and Japanese populations after administration of 20 mg of bitopertin in the age range of 20–70 years, with a proportion of females of 0.5, were 4.99 (95 % CI 4.81–5.17) L/h, 3.79 (95 % CI 3.66–3.91) L/h and 3.94 (95 % CI 3.80–4.10) L/h, respectively.
The predicted geometric mean CL/F values in Caucasians were 1.32-fold and 1.27-fold higher than the values in Chinese and Japanese, respectively. The body weight–normalized predicted geometric mean CL/F values in the Caucasian, Chinese and Japanese populations were 0.0670, 0.0624 and 0.0675 L/h/kg, respectively, which were very similar to each other.
3.4 Assessment of Ethnic Effects in Clearance, Using PopPK Analysis
A total of 1675 plasma concentration measurements (900 measurements from Caucasians, 478 from Chinese and 297 from Japanese) were used for population pharmacokinetic model development. The pharmacokinetics of bitopertin were best described by a two-compartment model incorporating a saturable Michaelis–Menten kinetic absorption process and dose-adjusted F a.
The data did not support the assignment of BSV to the maximum absorption rate constant (K a,Max), the dose with half of the maximum absorption rate constant (K a,50), the absorption lag time (t lag) and the dose with half of the absorbed fraction of the 3 mg dose (F a,50). The additive component was removed from the RUV because it was negligible.
BMI was identified as a covariate on the peripheral distribution volume (V 3/F). Body weight, BSA, age, sex and BMI had no significant effect on CL/F. Lastly, the effect of ethnicity was evaluated as a covariate on CL/F with a decrease of 6.03 in OFV. The estimated CL/F ratios of Chinese and Japanese to Caucasians were 0.833 and 0.858, respectively. Because the effect was not significant, it was not included in the final model. The estimated CL/F adjusted (CL/F adjusted = F a,relative × dose/AUC, where F a,relative is the F a of a different dose relative to 3 mg) and body weight–normalized CL/F adjusted in Caucasian, Chinese and Japanese populations can be seen in Fig. 1. Parameter estimates of the final model are summarized in Table 4.
The accuracy and robustness of the final model were evaluated using a VPC, and the final model adequately described the pharmacokinetic profile of bitopertin (Fig. 2).
Visual predictive checks of the final population pharmacokinetic model: results from 1000 simulated data sets. The blue circles represent observations. The solid red lines represent the 50th percentiles of the simulated profiles, and the dashed red lines represent the 5th and 95th percentiles. Conc concentration
3.5 Comparison of Predicted Clearance Values Between PBPK and PopPK Modelling
The predicted clearance values according to PBPK modelling and popPK analysis were compared using the demographics of the real studies. No same dose level was investigated in the three ethnic populations. The pharmacokinetic results after 10 and 20 mg, the dose levels that were used in phase 3 trials, were used for the comparison. The PBPK modelling–predicted bitopertin geometric mean CL/F values after administration of 20, 10 and 10 mg in Caucasian, Chinese and Japanese populations were 5.63, 3.79 and 5.03 L/h, respectively (Table 3). The PopPK analysis estimated CL/F adjusted values of 5.28 and 5.22 L/h without considering the effect of ethnicity for the 20 mg and 10 mg doses, respectively. The ratios of the geometric mean clearance values predicted by PBPK modelling to the CL/F adjusted estimated by popPK analysis in Caucasian, Chinese and Japanese populations were 1.07, 0.73 and 0.96, respectively. When ethnicity was incorporated as a covariate, the popPK analysis–estimated CL/F adjusted values in Caucasian, Chinese and Japanese populations were 5.55, 4.68 and 4.84 L/h, respectively. The ratios of the geometric mean clearance values predicted by PBPK modelling to the CL/F adjusted values estimated by popPK in Caucasian, Chinese and Japanese populations were 1.01, 0.81 and 1.04, respectively. The relatively larger difference in the ratio between the two methods in Chinese may have come from the additional correction of 0.85 that was used to estimate the liver weight in Chinese on the basis of BSA [9]. Further confirmation of liver weight in Chinese is required.
3.6 Comparison of Simulated Pharmacokinetic Profile Between PBPK and PopPK Modelling
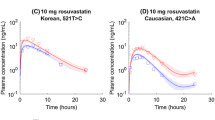
The simulated plasma concentration–time curves after oral administration of 20, 10 and 10 mg in Caucasian, Chinese and Japanese populations according to PBPK modelling and popPK analysis (using the model without ethnicity as a covariate) can be seen in Fig. 3. The plasma concentration–time curves simulated by PBPK modelling and popPK analysis (using the model without ethnicity as a covariate) were similar to each other and were in good agreement with the observed data. The comparisons of PBPK modelling and popPK analysis for bitopertin pharmacokinetic prediction can be seen in Table 5.
Simulated (lines) and observed (black circles) mean plasma concentration (Conc)–time curves (a, c, e) and absorption phase (b, d, f) of bitopertin in healthy Caucasians, Chinese and Japanese. The light grey lines represent the means of the simulations of individual trials (10 trials) according to physiologically based pharmacokinetic (PBPK) modelling. The solid black lines represent the means for the total virtual population according to PBPK modelling, and the dotted black lines represent the 5th and 95th percentiles. The dashed red lines represent the means of the simulations according to population pharmacokinetic analysis
4 Discussion
Assessing the sensitivity of a medicine’s PK/PD to ethnic factors is the key to extrapolating safety and efficacy from one region to another. Currently, in China and Japan, because of lack of understanding of the underlying mechanisms of ethnic differences, almost all drugs need to be assessed for ethnic sensitivity through conduct of bridging PK/PD studies, consuming significant amounts of resource and time. The current method for ethnic sensitivity assessment is empirical, molecule based and fragmental. It cannot be used to predict the ethnic sensitivity of NMEs. We propose a different strategy for NMEs (see Fig. 4: the right two triangles—NME focused), whereby bottom-up and top-down modelling approaches are applied in a sequential and complementary fashion. First, at the pre-clinical stage, mechanistic understanding of the molecule and its clearance pathways will serve as the input for PBPK modelling to predict ethnic sensitivity. The prediction will guide the early planning of ethnic bridging studies and/or assist dosing decision making and trial design, as well as directing enrolment of different ethnicities in phase 3 studies. Then, when pharmacokinetic data in multiple ethnicities are available, popPK analysis can be used to confirm the PBPK modelling results and provide confirmatory evidence for drug label. The bitopertin PBPK model was developed to simulate pharmacokinetics in Caucasian and Asian populations and to predict ethnic differences when there were only pharmacokinetic data in Caucasians. Subsequently, a popPK model was developed to assess the effects of ethnicity on pharmacokinetic parameters when human data from multiple ethnic groups were available to confirm the PBPK modelling results. The popPK model built on the basis of phase 1 data was consistent with the graphical analysis of the phase 3 data (unpublished data).
PBPK modelling predicted that the bitopertin geometric mean CL/F values after administration of 20 mg in the age range of 20–70 years would be 1.32-fold and 1.27-fold higher than the values in Chinese and Japanese, respectively. When ethnicity was incorporated as a covariate, the popPK analysis found that the ratios of typical clearance in Caucasians to the values in Chinese and Japanese were 1.20 and 1.17, respectively. The body weight–normalized CL/F was similar (Fig. 1). Thus, the popPK analysis results were similar to the PBPK modelling results.
For NMEs involving well-defined pharmacokinetic processes with well-established PBPK models, especially for those low-ethnic-sensitivity pathways that are defined, dedicated pharmacokinetic bridging studies may not be needed. For high-ethnic-sensitivity compounds, dose decisions may still need dedicated bridging studies. Subsequently, data from later-stage clinical trials can be used to build a popPK model to confirm the PBPK modelling results on ethnic differences, providing a more efficient way to address potential ethnic pharmacokinetic differences.
The well-established PBPK model for the CYP3A4 pathway ensured successful ethnic sensitivity prediction for bitopertin. For successful application of the proposed strategy on a broad scale, the ethnic-specific system data are yet to be collected and PBPK models of the major pharmacokinetic processes need to be validated. It is generally believed that PBPK models for CYP enzymes are reasonably well established [9, 10, 12], but the data on the gastrointestinal tract (e.g. enzymes, transporters, fluid dynamics), phase II metabolic enzymes and transporter-mediated pharmacokinetic pathways—and those data in special populations (e.g. paediatric subjects, and subjects with renal or hepatic impairment)—are still limited. We proposed that an academic–industry–regulatory consortium should be established to further investigate ethnic differences in absorption, distribution, metabolism and excretion (ADME) pathways and to enable improved PBPK modelling of the effects of ethnicity for a wide range of NMEs. The consortium would start by building a database of the effects of ethnicity on probe drugs for known clearance mechanisms (see Fig. 4: the left two triangles—pharmacokinetic-pathway focused). PopPK analysis can then be used as a screening tool to identify pharmacokinetic pathways that have significant ethnic differences. To probe the physiological factors causing the difference, PBPK models can be developed, refined and validated through a number of probe drugs with the same pharmacokinetic pathway, using full data sets of multiple ethnic groups. Once a set of such PBPK models is established for major pharmacokinetic processes, one can predict ethnic sensitivity for a given NME even with multiple pharmacokinetic pathways.
Bitopertin is an ideal probe drug for CYP3A4 because it is predominantly eliminated by hepatic CYP3A4 metabolism with a limited gut first-pass effect [7]. In this study, the ratios of typical clearance (not adjusted for body weight) in Caucasians to the values in Chinese and Japanese estimated by popPK analysis were 1.20 and 1.17, respectively. This was similar to the reported results for alprazolam, another CYP3A substrate [9]. The observed geometric mean alprazolam clearance value estimated using non-compartmental analysis in Caucasians was 1.27-fold higher than the value in Chinese. The body weight–normalized median alprazolam clearance values estimated using non-compartmental analysis were similar in Caucasians and Japanese [10]. This may indicate that the CYP3A4 activity in the liver in Caucasians was comparable to the values in Chinese and Japanese, which may be explained by the similar abundance of CYP3A4 in the three ethnic populations and can help to predict the ethnic sensitivity of CYP3A4 substrates (e.g. NMEs) in pharmacokinetics, using PBPK modelling. The difference in clearance due to hepatic blood flow and liver weight can be reflected in the body size. Therefore, the body weight–normalized clearance values for bitopertin were similar in the three ethnicities that were studied.
A more advanced absorption model (the advanced dissolution, absorption and metabolism [ADAM] model) has already been implemented in Simcyp®. In theory, it can be used to predict ethnic differences in absorption. However, the available physiological databases on ethnic differences in the gastrointestinal tract are very limited; thus, no relevant physiological differences exist in the Simcyp® models for different ethnicities. Therefore, we focus on the ethnic difference in clearance and choose the simple absorption model for bitopertin.
Although bitopertin PBPK models can capture most of the observed clearance values in Caucasians, Chinese and Japanese at different dose levels, it should be pointed out that data on the physiology, enzymes and transporters in Chinese and Japanese are still limited. More system data are needed and should be evaluated before the data are used for complex pharmacokinetic prediction. Ethnic differences in pharmacokinetics are just one component of ethnic sensitivity; comparability in disease biology, epidemiology and medical practice should be examined as well to ultimately elucidate ethnic differences in drug safety and efficacy.
5 Conclusion
PBPK modelling accurately predicted ~1.2-fold differences in the clearance of bitopertin between Caucasians and Asians without body weight adjustment, which was confirmed by the popPK analysis. As a general framework, PBPK modelling should be considered to predict ethnic sensitivity of pharmacokinetics prior to any human data and/or with data in only one ethnicity. Subsequently, popPK analysis can be used to confirm the effect of ethnicity and to support dose justification and labelling. PBPK modelling prediction and popPK analysis confirmation can complement each other for assessment of ethnic differences in clearance at different drug development stages. However, successful application of the strategy may be facilitated by an academic–industry–regulatory consortium to collect sufficient ethnic-specific system data and to validate PBPK models of the major pharmacokinetic processes.
References
Thiers FA, Sinskey AJ, Berndt ER. Trends in the globalization of clinical trials. Nat Rev Drug Discov. 2008;7:13–4.
Karlberg JPE. Globalization of sponsored clinical trials. Nat Rev Drug Discov. 2009;7:458–60.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline: ethnic factors in the acceptability of foreign clinical data E5(R1). ICH Secretariat. 1998. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E5_R1/Step4/E5_R1__Guideline.pdf. Accessed 26 Nov 2015.
Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–77.
Alberati D, Moreau JL, Lengyel J, Hauser N, Mory R, Borroni E, Pinard E, Knoflach F, Schlotterbeck G, Hainzl D, Wettstein JG. Glycine reuptake inhibitor RG1678: a pharmacologic characterization of an investigational agent for the treatment of schizophrenia. Neuropharmacology. 2012;62:1152–61.
Umbricht D, Alberati D, Martin-Facklam M, Borroni E, Youssef EA, Ostland M, Wallace TL, Knoflach F, Dorflinger E, Wettstein JG, Bausch A, Garibaldi G, Santarelli L. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71(6):637–46.
Parrott N, Hainzl D, Alberati D, Hofmann C, Robson R, Boutouyrie B, Martin-Facklam M. Physiologically based pharmacokinetic modeling to predict single- and multiple-dose human pharmacokinetics of bitopertin. Clin Pharmacokinet. 2013;52(8):673–83.
Parrott N, Hainzl D, Scheubel E, Krimmer S, Boetsch C, Guerini E, Martin-Facklam M. Physiologically based absorption modelling to predict the impact of drug properties on pharmacokinetics of bitopertin. AAPS J. 2014;16(5):1077–84.
Barter ZE, Tucker GT, Rowland-Yeo K. Differences in cytochrome P450-mediated pharmacokinetics between Chinese and Caucasian populations predicted by mechanistic physiologically based pharmacokinetic modeling. Clin Pharmacokinet. 2013;52:1085–100.
Inoue S, Howgate EM, Rowland-Yeo K, Shimada T, Yamazaki H, Tucker GT, Rostami-Hodjegan A. Prediction of in vivo drug clearance from in vitro data: II. Potential inter-ethnic differences. Xenobiotica. 2006;36(6):499–513.
Howgate EM, Rowland Yeo K, Proctor NJ, Tucker GT, Rostami-Hodjegan A. Prediction of in vivo drug clearance from in vitro data: I. Impact of inter-individual variability. Xenobiotica. 2006;36(6):473–97.
Feng S, Cleary Y, Parrott N, Hu P, Weber C, Wang Y, Yin OQ, Shi J. Evaluating a hysiologically based pharmacokinetic model for prediction of omeprazole clearance and assessing ethnic sensitivity in CYP2C19 metabolic pathway. Eur J Clin Pharmacol. 2015;71(5):617–24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Roche Postdoctoral Fellowship Programme.
Conflict of interest
Sheng Feng is a Roche Postdoctoral Fellow. Jun Shi, Neil Parrott, Cornelia Weber, Meret Martin-Facklam and Richard Peck are full-time employees of F. Hoffmann-La Roche. Pei Hu is a full-time employee of Peking Union Medical College Hospital. Tomohisa Saito is a full-time employee of Chugai Pharmaceutical Co., Ltd. The authors have no other conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Feng, S., Shi, J., Parrott, N. et al. Combining ‘Bottom-Up’ and ‘Top-Down’ Methods to Assess Ethnic Difference in Clearance: Bitopertin as an Example. Clin Pharmacokinet 55, 823–832 (2016). https://doi.org/10.1007/s40262-015-0356-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0356-1