Abstract
Background and Objectives
Oral enzalutamide (160 mg once daily) is approved for the treatment of metastatic castration-resistant prostate cancer (mCRPC). This article describes the pharmacokinetics of enzalutamide and its active metabolite N-desmethyl enzalutamide.
Methods
Results are reported from five clinical studies.
Results
In a dose-escalation study (n = 140), enzalutamide half-life was 5.8 days, steady state was achieved by day 28, accumulation was 8.3-fold, exposure was approximately dose proportional from 30–360 mg/day, and intersubject variability was ≤30 %. In a mass balance study (n = 6), enzalutamide was primarily eliminated by hepatic metabolism. Renal excretion was an insignificant elimination pathway for enzalutamide and N-desmethyl enzalutamide. In a food-effect study (n = 60), food did not have a meaningful effect on area under the plasma concentration–time curve (AUC) of enzalutamide or N-desmethyl enzalutamide, and in an hepatic impairment study, AUC of the sum of enzalutamide plus N-desmethyl enzalutamide was similar in men with mild (n = 6) or moderate (n = 8) impairment (Child–Pugh Class A and B) versus men with normal hepatic function (n = 14). In a phase III trial, an exposure-response analysis of steady-state predose (trough) concentrations (C trough) versus overall survival (n = 1103) showed that active treatment C trough quartiles for 160 mg/day were uniformly beneficial relative to placebo, and no threshold of C trough was associated with a statistically significant better response.
Conclusions
Enzalutamide has predictable pharmacokinetics, with low intersubject variability. Similar efficacy was observed in patients across the concentration/exposure range associated with a fixed oral dose of enzalutamide 160 mg/day.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This article summarizes data from several trials in order to provide clinical investigators with an understanding of the pharmacokinetics of enzalutamide. Collectively, the results show that enzalutamide has a half-life of 5.8 days, achieves steady state by day 28, accumulates 8.3-fold with once-daily dosing, shows approximate dose proportionality from 30–360 mg/day, and has ≤30 % intersubject variability. |
In addition, enzalutamide is primarily eliminated by hepatic metabolism, while renal excretion is an insignificant elimination pathway for enzalutamide and its active metabolite, N-desmethyl enzalutamide. Exposure to enzalutamide active moieties is not affected by food or baseline mild or moderate hepatic impairment. |
In an exposure-response analysis of overall survival in patients with mCRPC, active treatment C trough quartile groups for a fixed dose of 160 mg/day were uniformly beneficial relative to placebo, and there was no specific threshold of plasma concentrations in patients receiving enzalutamide that was associated with achieving a statistically significant better response; therefore, similar efficacy was observed in patients across the concentration/exposure range associated with a fixed oral dose of enzalutamide 160 mg/day. |
1 Introduction
Enzalutamide acts on multiple steps in the androgen receptor (AR) signaling pathway. It has been shown to competitively inhibit androgen binding to the AR, inhibit AR nuclear translocation, and inhibit AR interaction with DNA [1]. Enzalutamide decreased proliferation and induced cell death of prostate cancer cells in vitro and decreased tumor volume in a mouse prostate cancer xenograft model.
Enzalutamide (Xtandi®, Astellas Pharma US, Inc., Northbrook, IL, USA, and Medivation Inc., San Francisco, CA, USA) is approved in more than 40 countries for the treatment of patients with metastatic prostate cancer that has progressed on androgen deprivation therapy (gonadotropin-releasing hormone therapy or bilateral orchiectomy), a disease setting that is also defined as metastatic castration-resistant prostate cancer (mCRPC). The efficacy and safety of enzalutamide were assessed in randomized, placebo-controlled, multicenter, phase III clinical trials (AFFIRM and PREVAIL) [2, 3].
Enzalutamide (Fig. 1) is a small molecule with no ionizable groups at biologically relevant pH; therefore, enzalutamide solubility is not affected by pH over the physiological range. Enzalutamide exhibits limited aqueous solubility (≤2.0 μg/mL at relevant pH range), high permeability across Caco-2 monolayers (mean apparent permeability coefficient ≥31 × 10−6 cm/sec), and is not a substrate for P-glycoprotein (data on file, Medivation, Inc., 2012). Given its low solubility and high permeability, enzalutamide is considered a Biopharmaceutics Classification System (BCS) [4] class 2 drug substance.
The commercial product is a liquid-filled capsule of enzalutamide fully dissolved in caprylocaproyl polyoxylglycerides. This presentation was used throughout the development program. The approved dose is 160 mg (4 × 40 mg capsules) administered orally once daily with or without food.
Two major metabolites have been identified in human plasma, N-desmethyl enzalutamide and a carboxylic acid derivative (Fig. 1). Based on in vitro assays, N-desmethyl enzalutamide is an active metabolite that is thought to contribute to pharmacologic effects because it demonstrates key primary pharmacodynamics of similar potency to enzalutamide. In contrast, the carboxylic acid metabolite is inactive. At steady state, N-desmethyl enzalutamide circulates at approximately the same plasma concentration as enzalutamide, while the carboxylic acid metabolite is approximately 25 % lower.
The pharmacokinetics of enzalutamide were characterized in studies in patients with mCRPC, healthy male volunteers, and male subjects with impaired hepatic function. This article summarizes the data in order to provide clinical investigators with an understanding of the pharmacokinetics of enzalutamide.
2 Methods
2.1 Study Population
Pharmacokinetic results from clinical studies are summarized in this report: two studies in patients with mCRPC (n = 934), two in healthy male volunteers (n = 66), and one in healthy male volunteers (n = 14) and male subjects with impaired hepatic function (n = 14) (Table 1). The two studies in patients were assigned the following ClinicalTrials.gov registry numbers: NCT00510718 (dose-escalation study [5]) and NCT00974311 (phase III efficacy and safety study, AFFIRM [2]), while one of the studies in healthy volunteers was assigned the ClinicalTrials.gov registry number NCT01911715 (mass balance and biotransformation study) and the study in healthy volunteers and subjects with impaired hepatic function was assigned the registry number NCT01901133. The food-effect study in healthy volunteers was not assigned a registry number. All studies were conducted in accordance with the Declaration of Helsinki and with the approval of the appropriate local ethics committees. Informed consent was obtained from all subjects before study entry. Key eligibility criteria are summarized in Table 1, and the disease-specific criteria for the patient studies are described elsewhere [2, 5].
2.2 Pharmacokinetic and Analytical Methods
Pharmacokinetic blood samples were collected with dipotassium ethylenediaminetetraacetic acid (K2 EDTA) as the anticoagulant. Validated bioanalytical methods based on high-performance liquid chromatography with tandem mass spectrometry (LC–MS/MS) were used to analyze plasma, plasma dialysate, and urine samples for concentrations of enzalutamide and the two major human metabolites. The bioanalytical methods were validated in accordance with US FDA guidance [6]. In the method that supported plasma pharmacokinetic determinations in the phase I dose-escalation (first-in-man) trial, enzalutamide was analyzed over the concentration range of 0.002–5.0 µg/mL [electronic supplementary material (ESM) 1]. In the method that supported all subsequent studies, enzalutamide, N-desmethyl enzalutamide, and the carboxylic acid metabolite were simultaneously measured over the range of 0.02–50 µg/mL for each analyte [7].
Plasma concentrations within the validated concentration range were used to calculate pharmacokinetics parameters, which were estimated by non-compartmental methods using WinNonlin® (Pharsight Corporation, Palo Alto, CA, USA) and applicable complimentary software, such as SAS® (SAS Institute, Cary, NC, USA) and Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA). The estimated pharmacokinetics parameters included maximum plasma concentration (C max), time to C max (t max), trough (predose) plasma concentration (C trough), area under the plasma concentration–time curve (AUC) from time zero to the last quantifiable concentration (AUClast), AUC for one 24-h dosing interval at steady state (AUCτ), AUC from time zero to 24 h after administration of a single dose (AUC24h), AUC from time zero to infinity (AUC∞), terminal half-life (t ½), apparent oral clearance (CL/F), and apparent volume of distribution during the terminal phase (V z/F).
2.3 Study Design
2.3.1 Dose-Escalation Study
A phase I, open-label, dose-escalation safety and pharmacokinetics study of enzalutamide was performed in 140 patients with mCRPC [5]. Two patient populations were enrolled: chemotherapy-naïve patients (n = 65) and post-chemotherapy patients (n = 75). Patients received enzalutamide orally at 30, 60, 150, 240, 360, 480 (as 240 mg twice daily), and 600 mg (as 300 mg twice daily), depending on dosing cohort.
Full pharmacokinetic profiles for enzalutamide were collected from three or six patients per dose level during the single-dose period (with a full pharmacokinetics profile over an approximate 6-day period) and from all patients during the multiple-dose period (with a full pharmacokinetics profile on day 84 over a 24-h dosing interval). The pharmacokinetic sampling times during the single-dose period were as follows: predose and 0.5, 1, 2, 4, 6, 24, 48, 72, 96, and 120 h postdose. The pharmacokinetic sampling times during the multiple-dose period were as follows: predose on days 1, 3, 7, 28, 56, and 84, and postdose on day 84 at 0.5, 1, 2, 4, 6, 8, 12, and 24 h. In addition, predose C trough samples were collected from all patients on days 112, 140, and 168, and approximately every 4 weeks thereafter until discontinuation of enzalutamide. For the single-dose period, enzalutamide was taken in the clinic with breakfast. For the multiple-dose period, food intake was not controlled.
N-desmethyl enzalutamide and the carboxylic acid metabolite were not measured in this study because this was the first study in humans and the relative concentrations of these metabolites were not yet known.
Dose proportionality was assessed by a power model involving regression of logarithmically transformed AUC τ versus dose. A statistical test for dose proportionality was applied by determining if the slope was wholly contained within the 90 % confidence intervals (CI) of 0.800–1.25 and the p value for goodness of fit was ≤0.05.
2.3.2 Mass Balance and Biotransformation Study
A phase I, open-label, one-period, single-dose study in six healthy male subjects was performed to evaluate pharmacokinetics, metabolism, and excretion. On day 1, subjects received a single oral dose of enzalutamide 160 mg (4 × 40 mg capsules) plus an additional liquid-filled capsule containing a tracer dose of 14C-labeled enzalutamide (100 μCi) under fasting conditions. The radiochemical purity of 14C-enzalutamide was ≥99.7 %. Whole blood, plasma, urine, and feces were collected through day 77 postdose, and subjects were confined to the clinic up to day 11. After discharge, subjects visited the clinic for overnight stays (2 consecutive nights) on days 13–15, 20–22, 27–29, 34–36, 48–50, 62–64, and 76–78. During these visits, blood samples were taken, and urine and feces were collected for 24 h.
Details regarding the collection times for blood, plasma, urine, and feces, as well as methods used to calculate excretion parameters, can be found in ESM 2.
2.3.3 Food-Effect Study
A phase I, open-label, randomized, single-dose, parallel design, food-effect study was performed in healthy male subjects. The parallel design was considered appropriate because the t ½ of enzalutamide is long and intersubject variability in pharmacokinetics is low.
Subjects were randomized to receive a single oral dose of enzalutamide 160 mg (4 × 40 mg capsules) under fasting conditions (a minimum 10 h fast from food) or under fed conditions (n = 30 subjects per treatment arm). In the fed condition, subjects consumed a standard FDA high-fat, high-calorie breakfast containing 800–1000 calories, with approximately 50 % of this caloric content as fat [8] (the breakdown was approximately 150 calories from protein, 250 calories from carbohydrate, and 500–600 calories from fat). Blood samples for plasma pharmacokinetics were collected predose on day 1 and postdose through day 42 at the following times: on day 1 at 0.25, 0.5, 0.75, 1, 2, 3, 4, 6, 8, and 12 h, on day 2 at 0 and 12 h, and on days 3, 5, 7, 14, 21, 28, 35, and 42 at 0 h. These samples were analyzed for enzalutamide, N-desmethyl enzalutamide, and the carboxylic acid metabolite. The primary food-effect comparison was based on enzalutamide.
The food-effect analysis involved parallel group comparisons in which the fed condition (test) was compared with the fasted condition (reference). An analysis of variance was performed on the natural logarithmically transformed parameters AUClast, AUC∞, and C max using a mixed-effect model with food effect as the only factor.
2.3.4 Hepatic Impairment Study
A phase I, open-label, two-arm study was performed to determine the effect of mild and moderate hepatic impairment on the pharmacokinetics, safety, and tolerability of a single oral 160 mg dose of enzalutamide. Hepatic function was classified by the Child–Pugh system, as described by the FDA [9]. Arm A consisted of six subjects with mild hepatic impairment (Child–Pugh A, arm A1) and six matched control subjects with normal hepatic function (arm A2). Arm B consisted of eight subjects with moderate hepatic impairment (Child–Pugh B, arm B1) and eight control subjects with normal hepatic function (arm B2). The control subjects were individually matched for age (±5 years) and body mass index (BMI) (±15 %).
Each subject received a single oral dose of enzalutamide 160 mg, administered under fasting conditions. Blood samples for plasma pharmacokinetics were collected predose on day 1 and postdose through day 50 at the following times: on day 1 at 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, and 12 h; on days 2, 24, and 36 at 0 and 12 h; and on days 3, 4, 6, 8, 12, 15,19, 22, 26, 29, 36, 43, and 50 at 0 h. These samples were analyzed for enzalutamide, N-desmethyl enzalutamide, and the carboxylic acid metabolite. Plasma protein binding samples were collected at approximately 2, 96, and 120 h postdose, which corresponded to the expected t max for enzalutamide, the carboxylic acid metabolite, and N-desmethyl enzalutamide, respectively. The unbound fractions were determined by a validated method based on equilibrium dialysis.
Pharmacokinetic parameters were expressed in terms of total concentrations and (as appropriate) unbound concentrations. Statistical analyses compared subjects with mild hepatic impairment (arm A1, test) versus matched controls (arm A2, reference) and subjects with moderate hepatic impairment (arm B1, test) versus matched controls (arm B2, reference). The primary parameters of interest were the logarithmically transformed AUC∞ and C max. If the data did not permit estimation of AUC∞, then AUClast was used. All observations for test and reference were included in the statistical analysis, which used the MIXED procedure in SAS® with an unstructured covariance matrix. Treatment arm (A, B) and treatment group (test, reference) were included as fixed effects, and subject pair was included as a random effect. The least-square means (LSMs) of the primary parameters for each treatment group/arm combination were estimated, and a 90 % CI was constructed around the difference between the LSMs of test and reference for each arm.
2.3.5 Phase III Efficacy and Safety Study in Patients
The phase III AFFIRM trial [2] was a multinational, randomized, double-blind, placebo-controlled efficacy and safety study of oral enzalutamide in 1199 patients with mCRPC whose disease was progressing after one or two prior chemotherapy regimens, one of which was docetaxel-based. All patients continued androgen deprivation therapy. Patients were allowed, but not required, to take glucocorticoids. Patients were randomized 2:1 to receive enzalutamide 160 mg/day or matching placebo. Randomization was stratified by Eastern Cooperative Oncology Group Performance Status grade at baseline.
Predose C trough samples were collected from all patients at weeks 1, 2, 5, 9, 13, and 25, and every 12 weeks thereafter to assess plasma concentrations of enzalutamide, N-desmethyl enzalutamide, and the carboxylic acid metabolite. Based on t ½ estimates, enzalutamide was expected to be at steady state by week 5, and the metabolites were expected to be at steady state by week 9.
An exposure–response analysis was performed to assess relationships between plasma exposure of the sum of enzalutamide plus N-desmethyl enzalutamide versus the efficacy endpoint of overall survival. The purpose of this analysis was to look for trends in the data that might shed light on the suitability of a fixed oral dose of 160 mg/day. The source data, endpoint derivation, and stratification were as described previously [2], and the intent-to-treat population was as described in ESM 3. At the time of the data cutoff for the analysis for overall survival, the median time on study drug was 8.3 months for enzalutamide and 3.0 months for placebo. Exposure for each patient was defined as his mean steady-state C trough value (i.e. the mean concentration for all predose samples that were collected after at least 56 days of consecutive dosing at a particular dose of enzalutamide). The C trough values were evaluated as continuous and discrete parameters. When evaluated as discrete parameters, the C trough values were classified into quartiles that divided the derived non-zero exposure parameters into four approximately equal groups, from lowest exposure quartile (Q1) to highest exposure quartile (Q4), after sorting by rank order. Exposure categories included the placebo‐randomized patients as one category and four categories (quartiles) of exposure for the enzalutamide‐randomized patients. Patients randomized to placebo were assigned a C trough value of zero.
Log‐rank tests for homogeneity assessed differences between Kaplan–Meier exposure category curves, and pairwise log‐rank tests compared exposure categories with one another. In addition, a Cox proportional hazard analysis of C trough as a continuous variable assessed the association between exposure and event risk. Where a statistically significant slope was observed, pairwise Cox proportional hazard estimates compared exposure categories. Statistical significance was assigned using a nominal two‐sided type I error rate of 5 %, with no adjustments made for multiplicity.
3 Results
3.1 Participants
Demographics and baseline characteristics of subjects in the five studies are summarized in Table 2.
3.2 Dose-Escalation Study
In the single-dose period, enzalutamide was absorbed rapidly after a single oral dose, with a median t max of 1 h (range 0.4–4.0 h) (Table 3). The t ½, CL/F, and V z/F values were not affected by dose size.
In the multiple-dose period, enzalutamide was absorbed rapidly on day 84, with the median t max ranging from 0.00 to 2.07 h (Table 4), which was similar to the median tmax in the single-dose period. The mean CL/F was 0.61 L/h, also similar to the CL/F during the single-dose period. In general, dosing for 1 month was required to reach steady state, and the daily fluctuations in plasma concentrations were low (mean peak-to-trough ratio, 1.25). As a result of the low daily fluctuations, plasma profiles at steady state resembled a constant infusion. The enzalutamide C trough values in individual patients remained constant (i.e. did not show an upward or downward trend over time) beyond 1 month of long-term therapy, demonstrating time-linear pharmacokinetics once steady state was achieved. The coefficient of variation (standard deviation/mean) for AUC τ , C trough, and C max was ≤30 %, demonstrating low intersubject variability. With daily oral administration, enzalutamide accumulated approximately 8.3-fold relative to the single dose. C max increased with increasing dose, with the exception of 480 and 600 mg, which were given as a divided dose (240 and 300 mg twice daily, respectively), with approximately 12 h between the two doses. To assess dose-proportionality of AUC, a power model was applied to AUC τ values for doses ranging from 30 to 360 mg/day (insufficient data were available at 480 and 600 mg/day). The estimated slope was 0.872 with a 90 % CI of 0.799–0.944, and the p-value for goodness of fit was 0.0043 (Fig. 2). As the 90 % CI of the slope was mostly contained within the interval of 0.800–1.25, and the p value for the goodness of fit was ≤0.05, the analysis indicated no major deviations from dose proportionality.
Dose-proportionality assessment of enzalutamide for doses ranging from 30 to 360 mg/day. A linear regression (power model) analysis was applied to the mean values for the AUCτ after approximately 3 months of treatment. Circles denote individual patients and the gray area depicts 90 % CIs. AUC τ area under the plasma concentration–time curve during one 24-h dose interval at steady state, CIs confidence intervals
3.3 Mass Balance and Biotransformation Study
Enzalutamide was absorbed rapidly after oral administration, with a median t max of 1.75 h (range 1–3 h; Table 5). The mean t ½ of enzalutamide in plasma was 69.8 h (2.9 days). N-desmethyl enzalutamide and the carboxylic acid metabolite appeared to be formed slowly, with median t max in plasma of 132 and 96 h (5.5 and 4.0 days) postdose, respectively. The mean t ½ of N-desmethyl enzalutamide and the carboxylic acid metabolite in plasma were 186 and 223 h (7.8 and 9.3 days), respectively.
The mean concentration-time profiles of 14C-concentrations in plasma and whole blood were essentially parallel (Fig. 3). The overall whole blood to plasma ratio was 0.55, indicating that the radioactivity was preferentially retained in the plasma component of blood. Enzalutamide, N-desmethyl enzalutamide, and the carboxylic acid metabolite accounted for 88 % of the 14C-radioactivity in plasma, representing 30, 49, and 10 % of the total AUC, respectively. In consideration of these percentages, N-desmethyl enzalutamide and the carboxylic acid metabolite are major human metabolites.
The overall mean total recovery of 14C-radioactivity in urine and feces was 84.6 % of the administered dose (Fig. 4). The major route of excretion of 14C-radioactivity was urine (71.0 % of dose), primarily as metabolites. The remainder of the radioactivity was excreted in feces (13.6 % of dose). Based on LC-MS/MS analysis, the main component in urine was the carboxylic acid metabolite, which accounted for 62.7 % of the dose. A trace amount of unchanged parent enzalutamide was excreted in urine. N-desmethyl enzalutamide was too low to quantitate in urine by LC–MS/MS. Based on this information, renal excretion is a minor elimination pathway for unchanged parent enzalutamide and N-desmethyl enzalutamide.
Mean cumulative 14C-radioactivity recovery–time profiles after a single oral dose of 14C-enzalutamide (160 mg, 100 µCi) in the mass balance and biotransformation study(n = 6 healthy adult males). The majority of excretion of drug or drug-related product in urine was in the form of carboxylic acid metabolite
In feces, 0.39 % of the dose was recovered as unchanged parent enzalutamide. Given that overall recovery in excreta was 84.6 % of the dose, at least 84.2 % of the dose was absorbed.
3.4 Food-Effect Study
Enzalutamide was absorbed rapidly after oral administration, with a median t max of 1 and 2 h postdose for the fasted and fed conditions, respectively (Table 6).
Based on AUC values for enzalutamide (Table 6), the extent of absorption was similar after a high-fat meal and under fasting conditions. The geometric mean AUC values were approximately 1 % lower in fed than in fasted subjects, and the 90 % CI for the ratio of treatment mean AUC values were within the 0.80–1.25 range, establishing the absence of a food effect. Based on C max and t max values, the rate of absorption after a high-fat meal was slower than under fasting conditions. The geometric mean C max was approximately 30 % lower and median t max was approximately 1 h later with food. The 90 % CI for the ratio of treatment mean C max values was 0.63–0.78, which is below the 0.80–1.25 range to establish absence of a food effect. Taken together, the results show that there was a negligible change in AUC and a slightly lower C max when enzalutamide was taken after a high-fat meal. These C max changes are not considered clinically relevant.
Summary statistics for N-desmethyl enzalutamide (Table 6) show that food does not have a clinically meaningful effect on exposure to the active metabolite.
3.5 Hepatic Impairment Study
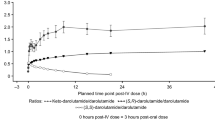
The mean plasma concentration–time profiles for the sum of enzalutamide plus N-desmethyl enzalutamide after single-dose oral administration of enzalutamide 160 mg in subjects with mild or moderate hepatic impairment and in matched control subjects are presented in Fig. 5. Exposure parameters (AUC∞, C max) for enzalutamide, N-desmethyl enzalutamide, and the sum of enzalutamide plus N-desmethyl enzalutamide were no more than 1.30-fold higher in subjects with mild or moderate hepatic impairment than in matched control subjects with normal hepatic function (Table 7). Exposure parameters (AUClast, C max) for the carboxylic acid metabolite were unchanged with mild hepatic impairment and tended to be approximately 40 % lower in subjects with moderate hepatic impairment.
Concentration–time profiles (mean ± standard deviation) for the sum of enzalutamide plus N-desmethyl enzalutamide after a single oral dose of enzalutamide 160 mg in male subjects: a subjects with mild hepatic impairment (Child–Pugh Class A; n = 8) and the age- and BMI-matched control subjects with normal hepatic function (n = 8); b subjects with moderate hepatic impairment (Child–Pugh Class B; n = 6) and the age- and BMI-matched control subjects with normal hepatic function (n = 6). BMI body mass index
The mean values for CL/F were similar in subjects with normal hepatic function, mild hepatic impairment, and moderate hepatic impairment (Table 5). The mean values for V z/F and t ½ were similar in subjects with normal hepatic function and mild hepatic impairment, while in subjects with moderate hepatic impairment, the mean values for V z/F and t ½ were approximately twofold higher. The mean unbound fraction for enzalutamide ranged from 1.49 to 2.39 %, from 2.62 to 3.63 % for N-desmethyl enzalutamide, and from 1.43 to 2.28 % for the carboxylic acid metabolite. The mean values for the unbound fractions were similar in subjects with normal hepatic function and subjects with impaired hepatic function, and there were no trends in the data to suggest alterations in the unbound fraction with mild or moderate hepatic impairment for any of the three molecules. Comparisons of AUC and C max based on the unbound concentrations of enzalutamide and its metabolites produced essentially the same ratios as AUC and C max based on total concentrations.
3.6 Phase III Efficacy and Safety Study
Pharmacokinetic assessments in patients taking enzalutamide 160 mg/day were based on predose C trough samples. Beyond week 5 (for enzalutamide) and week 9 (for the metabolites), the steady-state C trough values in individual patients remained constant (i.e. no general upward or downward trends with time) during more than 1 year of long-term therapy (Fig. 6), demonstrating time-linear pharmacokinetics once steady state was achieved. At week 13, the mean ± standard deviation C trough for enzalutamide was 11.4 ± 2.95 μg/mL (n = 679), 13.0 ± 3.78 μg/mL (n = 680) for N-desmethyl enzalutamide, and 8.44 ± 6.77 μg/mL (n = 680) for the carboxylic acid metabolite. Intersubject variability in C trough values for enzalutamide and N-desmethyl enzalutamide were ≤29 percent coefficient of variation (%CV), and 80 %CV for the carboxylic acid metabolite.
C trough versus time profiles for enzalutamide, N-desmethyl enzalutamide, and the carboxylic acid metabolite in an mCRPC patient taking enzalutamide 160 mg/day in the phase III trial (AFFIRM) [2]. C trough trough plasma concentration (measured concentration in a predose sample taken directly before the next administration), mCRPC metastatic castration-resistant prostate cancer
The exposure-response analysis examined the relationships between C trough quartiles for the sum of enzalutamide plus N-desmethyl enzalutamide and the efficacy endpoint of overall survival (ESM 3). The Kaplan–Meier exposure–response curves of overall survival for the composite sum of enzalutamide plus N-desmethyl enzalutamide are shown in Fig. 7. In all pairwise comparisons versus placebo, the effects of the active treatment C trough quartile groups were statistically significant (p ≤ 0.0001) in favor of active treatment, supporting the conclusion that the enzalutamide treatment was statistically superior to placebo for overall survival (Fig. 8). In addition, all assessments of overall survival versus the sum of enzalutamide plus N-desmethyl enzalutamide C trough values as a continuous variable resulted in statistically significant slopes (p < 0.0001). Although this suggests an association between higher levels of exposure and improved prognosis for patients, pairwise tests showed no difference in the risk of events among active treatment C trough quartiles (p ≥ 0.5499) (Fig. 7). Thus, the active treatment C trough quartile groups were uniformly beneficial relative to placebo, and there was no specific threshold of plasma concentrations in patients receiving enzalutamide that was associated with achieving a statistically significant better response. Similar results were obtained for exposure–response analyses based on C trough values for enzalutamide alone and N-desmethyl enzalutamide alone. Overall, the exposure‐response analysis showed similar efficacy in patients across the concentration/exposure range associated with a fixed oral dose of enzalutamide 160 mg/day.
Kaplan–Meier exposure–response analysis for exposure to enzalutamide plus N-desmethyl enzalutamide versus overall survival in the intent-to-treat population in the phase III clinical trial (AFFIRM) [2]. Exposure was based on time‐averaged steady-state predose (C trough) plasma concentrations that were classified into quartiles. The analysis involved 1103 patients (n = 176 patients in each of Q1, Q2, Q3, and Q4, and n = 399 placebo patients). C trough trough plasma concentration, Q exposure quartile
4 Discussion
Combined pharmacokinetic data from patients and healthy subjects provided insights into the absorption, distribution, and elimination properties of enzalutamide. With regard to absorption, after single- and multiple-dose oral administration, the t max generally occurred around 1 h postdose, showing that enzalutamide is rapidly absorbed. Based on excretion of metabolites in urine and feces in the mass balance and biotransformation study, the extent of absorption of enzalutamide after oral administration is at least 84.2 %. With regard to distribution, the mean V z/F of enzalutamide in patients (110 L) was approximately 2.6 times greater than the volume of total body water (42 L [10]), and approximately 37 times greater than the plasma volume (3 L [10]), suggesting extensive extravascular distribution of the drug. With regard to elimination, urinary and fecal recovery data after oral administration of 14C-enzalutamide showed that enzalutamide is primarily eliminated by hepatic metabolism, and renal excretion is an important pathway for the carboxylic acid metabolite and an insignificant elimination pathway for unchanged parent enzalutamide and N-desmethyl enzalutamide. The mean CL/F of enzalutamide was 0.56 L/h in patients, corresponding to approximately 1 % of liver plasma flow (48.7 L/h [10]); thus, enzalutamide is a low extraction ratio drug.
With daily oral administration, enzalutamide achieved steady state in patients by day 28, and accumulated approximately 8.3-fold relative to a single dose, which is consistent with the long t ½ (5.8 days). Due to the long t ½, daily fluctuations in plasma concentrations were low (mean peak-to-trough ratio, 1.25), and enzalutamide therefore had a relatively flat concentration profile over the dose interval with once-daily dosing. The steady-state C trough values for enzalutamide and N-desmethyl enzalutamide in individual patients remained constant during more than 1 year of long-term therapy, demonstrating time-linear pharmacokinetics once steady state was achieved. At steady state, enzalutamide showed no major deviations from dose proportionality over the daily dose range of 30–360 mg. In patients, %CV for AUCτ, C trough, and C max was ≤30 %, demonstrating low intersubject variability.
Consumption of a high-fat meal did not affect the extent of enzalutamide absorption but did result in a slower rate of absorption, as evidenced by a geometric mean C max that was approximately 30 % lower and a median t max that was approximately 1 h later. Given the long t ½ (5.8 days) and low peak-to-trough ratio (1.25) with once-daily dosing, these changes in the rate of absorption have essentially no effect on the pharmacokinetic profile of enzalutamide under conditions of clinical use; therefore, enzalutamide can be taken with or without food.
Mild hepatic impairment had no impact on the pharmacokinetics of enzalutamide or its major metabolites. Moderate hepatic impairment had no impact on exposure parameters (AUC∞ and C max) for enzalutamide active moieties (enzalutamide and N-desmethyl enzalutamide) but was associated with a decrease of approximately 40 % in exposure parameters for the carboxylic acid, which is of no clinical importance because this metabolite is inactive. Subjects with moderate hepatic impairment showed no apparent change in CL/F for enzalutamide but did demonstrate an approximately twofold increase in V z/F and t ½. The reason for the increase in V z/F is unknown but may be attributed to a combination of factors typically associated with hepatic impairment, namely decreased concentrations of serum albumin, increased extracellular fluid (due to ascites and edema), and decreased muscle mass. As t ½ is determined by the ratio of V z/F to CL/F, the increase in t ½ is attributed to the increase in V z/F. Importantly, as the composite AUC∞ and C max of enzalutamide plus N-desmethyl enzalutamide were similar in subjects with mild or moderate hepatic impairment relative to subjects with normal hepatic function, no starting dose adjustment is needed when treating patients with mild or moderate hepatic impairment with enzalutamide. The effects of baseline severe hepatic impairment (Child–Pugh Class C) on enzalutamide pharmacokinetics have not yet been assessed.
Based on the results of the phase III trial in patients with mCRPC, there is no apparent exposure–response relationship for the efficacy endpoint of overall survival within a single fixed oral dose of 160 mg/day. As there was no specific threshold of plasma concentration in patients receiving enzalutamide that was associated with achieving a statistically significant better response, the analysis supports the suitability of a fixed oral dose of enzalutamide 160 mg/day for the treatment of patients with mCRPC.
5 Conclusions
Data from the studies summarized in this paper show that orally administered enzalutamide has a predictable pharmacokinetic profile with low intersubject variability, and similar efficacy is observed in patients across the concentration/exposure range associated with a fixed oral dose of enzalutamide 160 mg/day.
References
Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–90.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, AFFIRM Investigators, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate before chemotherapy. N Engl J Med. 2014;371(5):424–33.
US Department of Health and Human Services. Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. 2000. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070246. Accessed 13 Feb 2015.
Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium. Antitumour activity of enzalutamide in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;24;375(9724):1437–46.
US Department of Health and Human Services. Bioanalytical method validation. 2001. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107. Accessed 13 Feb 2015.
Bennett D, Gibbons JA, Mol R, Ohtsu Y, Williard C. Validation of a method for quantifying enzalutamide and its major metabolites in human plasma by LC–MS/MS. Bioanalysis. 2014;6(6):737–44.
US Department of Health and Human Services. Food-effect bioavailability and fed bioequivalence studies. 2003. Available at: http://www.fda.gov/RegulatoryInformation/Guidances/UCM126833. Accessed 13 Feb 2015.
US Department of Health and Human Services. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. 2005. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072123. Accessed 13 Feb 2015.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Acknowledgments
Jacqueline Gibbons and Joyce Mordenti are employees of Medivation, Inc, and Vanessa Beddo is an employee of ICON Clinical Research. Yoshiaki Ohtsu is an employee of Astellas Pharma, Inc. All other authors are employees of Astellas Pharma Europe B.V. The research described in this study was funded by Medivation, Inc. and Astellas Pharma, Inc., and individuals from these organizations were involved in the design and conduct of the study, collection of the data, and analysis and interpretation of the data. Copy editing assistance was provided by Shannon Davis of Infusion Communications and funded by Medivation, Inc. The authors take full responsibility for the content of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. A. Gibbons and T. Ouatas contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gibbons, J.A., Ouatas, T., Krauwinkel, W. et al. Clinical Pharmacokinetic Studies of Enzalutamide. Clin Pharmacokinet 54, 1043–1055 (2015). https://doi.org/10.1007/s40262-015-0271-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0271-5