Abstract
Background and Objectives
Abiraterone acetate, an androgen biosynthesis inhibitor, prolongs survival in men with metastatic castration-resistant prostate cancer (mCRPC) in the pre- and post-chemotherapy setting as demonstrated by the pivotal phase III studies COU-AA-301 and COU-AA-302. We performed population pharmacokinetic analyses to estimate pharmacokinetic parameters after oral administration of 1,000 mg/day of abiraterone acetate in patients with mCRPC, with or without prior chemotherapy, and after a single 1,000 mg dose in healthy volunteers. The study objectives were to determine consistency between patient populations and to characterize factors that may influence abiraterone pharmacokinetics.
Methods
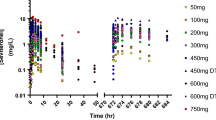
Studies in this analysis included COU-AA-302 (chemotherapy naïve); COU-AA-301 and COU-AA-006 (chemotherapy pretreated); and COU-AA-008, COU-AA-009, and COU-AA-014 (healthy subjects). A total of 4,627 plasma concentrations from 359 subjects (62 healthy volunteers, 297 patients) were analyzed using non-linear mixed-effects modeling.
Results
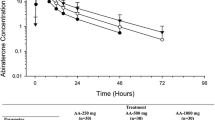
An Erlang-type absorption model with first-order elimination and three-transit compartments following sequential zero- and first-order processes was used to characterize abiraterone pharmacokinetics. Absorption-related parameters were affected by food intake. Abiraterone pharmacokinetics were characterized by an extensive apparent clearance, which was lower in patients with mCRPC (1,550 L/h) versus healthy subjects (2,240 L/h), and by large apparent central (5,620 L) and peripheral (17,400 L) volumes of distribution. Abiraterone pharmacokinetics were similar in chemotherapy-pretreated and -naïve patients and were characterized by a high between- and within-subject variability [e.g., between-subject coefficient of variation (CV%) for relative bioavailability for the modified fasting state was 61.1 % and the CV% for within-subject variability was 71.3 %]. The fat content of food taken with abiraterone acetate affected the bioavailability of abiraterone. No factors beyond food intake and health status (healthy vs. mCRPC) impacted abiraterone pharmacokinetics.
Conclusions
Based on the pharmacokinetics model, the recommended 1,000 mg/day of abiraterone acetate resulted in similar abiraterone exposure for patients with mCRPC regardless of prior chemotherapy. The fat content of food affected relative bioavailability of abiraterone, though the extent of this effect is dependent on health status.





Similar content being viewed by others
References
Acharya M, Gonzalez M, Mannens G, et al. A phase I, open-label, single-dose, mass balance study of 14C-labeled abiraterone acetate in healthy male subjects. Xenobiotica. 2013;43:379–89.
Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48.
Yap TA, Carden CP, Attard G, et al. Targeting CYP17: established and novel approaches in prostate cancer. Curr Opin Pharmacol. 2008;8:449–57.
Ang JE, Olmos D, de Bono JS. CYP17 blockade by abiraterone: further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br J Cancer. 2009;100:671–5.
Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71.
Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–8.
Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–8.
Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92.
Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–501.
Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–95.
Auchus RJ. The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinol Metab Clin North Am. 2001;30:101–19 vii.
ZYTIGA® (abiraterone acetate) [US prescribing information]. Horsham: Janssen Biotech Inc.; 2013.
Acharya M, Bernard A, Gonzalez M, et al. Open-label, phase I, pharmacokinetic studies of abiraterone acetate in healthy men. Cancer Chemother Pharmacol. 2012;69:1583–90.
Acharya M, Bernard A, Griffin T, et al. A phase 1 study to determine the effect of food on the pharmacokinetics of abiraterone acetate (AA) in healthy male subjects [abstract no. T3381]. Annual Meeting of American Association of Pharmaceutical Scientists; 23–27 Oct 2011; Washington, DC.
Goldberg T, Berrios-Colon E. Abiraterone (zytiga), a novel agent for the management of castration-resistant prostate cancer. P T. 2013;38:23–6.
Tolcher AW, Chi KN, Shore ND, et al. Effect of abiraterone acetate plus prednisone on the QT interval in patients with metastatic castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2012;70:305–13.
Viswanathan CT, Bansal S, Booth B, et al. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm Res. 2007;24:1962–73.
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). Guidance for industry: bioanalytical method validation. Food and Drug Administration. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf. Accessed 12 Aug 2014.
Xu XS, Dunne A, Kimko H, et al. Impact of low percentage of data below the quantification limit on parameter estimates of pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2011;38:423–32.
Preston SL, Drusano GL, Berman AL, et al. Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob Agents Chemother. 1998;42:1098–104.
Savic RM, Karlsson MO. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11:558–69.
Xu XS, Yuan M, Karlsson MO, et al. Shrinkage in nonlinear mixed-effects population models: quantification, influencing factors, and impact. AAPS J. 2012;14:927–36.
Bonate PL. Nonlinear mixed effects models: theory. In: Pharmacokintic-pharmacodynamic modeling and simulation. New York: Springer; 2006: 205.
Bruno R, Vivier N, Vergniol JC, et al. A population pharmacokinetic model for docetaxel (Taxotere): model building and validation. J Pharmacokinet Biopharm. 1996;24:153–72.
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–12.
Savic RM, Jonker DM, Kerbusch T, et al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34:711–26.
Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–36.
Loos WJ, Gelderblom H, Sparreboom A, et al. Inter- and intrapatient variability in oral topotecan pharmacokinetics: implications for body-surface area dosage regimens. Clin Cancer Res. 2000;6:2685–9.
Hande K, Messenger M, Wagner J, et al. Inter- and intrapatient variability in etoposide kinetics with oral and intravenous drug administration. Clin Cancer Res. 1999;5:2742–7.
Mathijssen RH, Sparreboom A, Verweij J. Determining the optimal dose in the development of anticancer agents. Nat Rev Clin Oncol. 2014;11:272–81.
US Food and Drug Administration. Drug development and drug interactions: table of substrates, inhibitors, and inducers. Food and Drug Administration. http://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm093664.htm#cypEnzymes. Accessed 31 Oct 2013.
Cheeti S, Budha NR, Rajan S, et al. A physiologically based pharmacokinetic (PBPK) approach to evaluate pharmacokinetics in patients with cancer. Biopharm Drug Dispos. 2013;34:141–54.
Ramalingam SS, Kummar S, Sarantopoulos J, et al. Phase I study of vorinostat in patients with advanced solid tumors and hepatic dysfunction: a National Cancer Institute Organ Dysfunction Working Group study. J Clin Oncol. 2010;28:4507–12.
Marbury T, Lawitz E, Stonerock R, et al. Single-dose pharmacokinetic studies of abiraterone acetate in men with hepatic or renal impairment. J Clin Pharmacol. 2014;54:732–41.
US Food and Drug Administration. Blood serum chemistry—normal values. Food and Drug Administration: Investigators Operations Manual. http://www.fda.gov/downloads/ICECI/Inspections/IOM/UCM135835.pdf Accessed 11 Aug 2014.
Chi KN, Spratlin J, Kollmannsberger C, et al. Evaluation of continuous abiraterone acetate plus prednisone dosing in fasting and fed states in patients with metastatic castration-resistant prostate cancer [abstract no. P408]. European Cancer Congress; 27 Sep–1 Oct 2013; Amsterdam.
Xu XS, Ryan CJ, Stuyckens K, et al. Relationship between abiraterone exposure, prostate-specific antigen (PSA) kinetics, and overall survival (OS) in metastatic castration-resistant prostate cancer (mCRPC) patients. American Society of Clinical Oncology Genitourinary Cancers Symposium; 30 Jan–1 Feb 2014; San Francisco, CA, USA.
Gravanis I, Lopez AS, Hemmings RJ, et al. The European medicines agency review of abiraterone for the treatment of metastatic castration-resistant prostate cancer in adult men after docetaxel chemotherapy and in chemotherapy-naive disease: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist. 2013;18:1032–42.
Acknowledgments
This study was sponsored by Janssen Research & Development, Raritan, NJ, USA. Writing assistance was provided by Shala Thomas, PhD, of PAREXEL, and was funded by Janssen Global Services, LLC. The authors wish to also thank Sandra Boom (Pharma-Plus) for her assistance in the preparation of this manuscript, funded by Janssen Research & Development.
Disclosures
K. Stuyckens, X.S. Xu, T.W. Griffin, M. Yu, A. Vermeulen, P. Nandy, and I. Poggesi are employees of Janssen Research & Development and own stock in Johnson & Johnson. F. Saad has served as a consultant and speaker for and has received research funding from Janssen Research & Development. C. Ryan has served as a speaker for and has received research funding from Janssen Research & Development. M. Smith has served as a consultant for and has received research funding from Janssen Research & Development.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stuyckens, K., Saad, F., Xu, X.S. et al. Population Pharmacokinetic Analysis of Abiraterone in Chemotherapy-Naïve and Docetaxel-Treated Patients with Metastatic Castration-Resistant Prostate Cancer. Clin Pharmacokinet 53, 1149–1160 (2014). https://doi.org/10.1007/s40262-014-0178-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0178-6