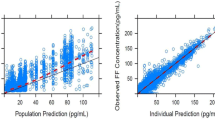
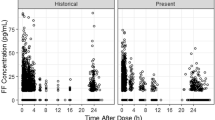
Abstract
Background and Objectives
PF-00610355 is an orally inhaled long-acting β2-adrenoreceptor agonist that is being developed for the once-daily treatment of chronic obstructive pulmonary disease (COPD). The pharmacological effect is exerted in the lungs. However, systemic exposure of PF-00610355 is expected to be responsible for certain drug-related adverse effects. This analysis characterizes PF-00610355 using an integrated analysis of systemic exposure, across trials and patient populations.
Methods
A total of 6,107 samples of PF-00610355 plasma concentration, collected in 264 subjects from eight studies in healthy volunteers, asthma, and COPD patients, were analyzed using non-linear mixed-effects models. Model-based mean (95 % CI) exposure profiles for a range of PF-00610355 doses in COPD patients were simulated.
Results
PF-00610355 exposure profiles were described by a three-compartment disposition model with first-order absorption through a transit compartment. Patient status, inhalation device, and demographic factors were found to influence systemic drug exposure. Relative fine particle dose had a minor effect, whereas no effect of baseline lung function on the systemic exposure was found. An implicit method to address pharmacokinetic variability between occasions of drug intake yielded similar results as the established explicit method, yet in a much more efficient way.
Conclusion
The estimated systemic pre-dose and maximum PF-00610355 plasma concentration was 23 and 38 % in COPD patients compared to healthy volunteers, respectively. The analysis illustrated the value of an integrated pharmacokinetic analysis to address specific challenges in the clinical development of long-/ultra-long-acting β2-agonists and inhaled compounds in general, both in relation to selecting a safe starting dose in patients, but also in understanding exposure and systemic safety information across different patient populations and different inhalation devices/formulations.




Similar content being viewed by others
References
Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76.
Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815–21.
Rossi A, Kristufek P, Levine BE, et al. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058–69.
Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA). BMJ. 2000;320:1368–73.
Beier J, Beeh KM. Long-acting beta-adrenoceptor agonists in the management of COPD: focus on indacaterol. Int J Chron Obstruct Pulmon Dis. 2011;6:237–43.
Glossop PA, Lane CA, Price DA, et al. Inhalation by design: novel ultra-long-acting β(2)-adrenoreceptor agonists for inhaled once-daily treatment of asthma and chronic obstructive pulmonary disease that utilize a sulfonamide agonist headgroup. J Med Chem. 2010;53(18):6640–52.
Weda M, Zanen P, de Boer AH, et al. The therapeutic index of locally acting inhaled drugs as a function of their fine particle mass and particle size distribution: a literature review. Curr Drug Deliv. 2008;5:142–7.
Diderichsen PM, Cox E, Martin SW, et al. Predicted heart rate effect of inhaled PF-00610355, a long acting beta-adrenoceptor agonist, in volunteers and patients with chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2013. doi:10.1111/bcp.12080.
Savic RM, Karlsson MO. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11:558–69.
Karlsson MO, Sheiner LB. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm. 1993;21:735–50.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57.
R Development Core Team. R: a language and environment for statistical computing. http://www.R-project.org; 2011.
Savic RM, Jonker DM, Kerbusch T, et al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34:711–26.
Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6(1):67–74.
Matsushima S, Matthews I, Woessner R, Pinault G, Hara H, Wilkins J, Sekiguchi K. Systemic pharmacokinetics of indacaterol, an inhaled once-daily long-acting beta2-agonist, in different ethnic populations. Int J Clin Pharmacol Ther. 2012;50:545–56.
Bennett JA, Harrison TW, Tattersfield AE. The contribution of the swallowed fraction of an inhaled dose of salmeterol to it systemic effects. Eur Respir J. 1999;13:445–8.
Acknowledgments
The analysis and preparation of the publication were funded by Pfizer Inc. Dr Cox and Dr Diderichsen contributed to the analysis and preparation of the publication as consultants paid by Pfizer Inc. The authors report no relevant conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Diderichsen, P.M., Cox, E., Martin, S.W. et al. Characterizing Systemic Exposure of Inhaled Drugs: Application to the Long-Acting β2-Agonist PF-00610355. Clin Pharmacokinet 52, 443–452 (2013). https://doi.org/10.1007/s40262-013-0048-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-013-0048-7