Abstract
Objective
Since May 2018, a 6-year post‑marketing surveillance (PMS) has been underway to evaluate the safety and effectiveness of letermovir for cytomegalovirus (CMV) prophylaxis in Japanese patients with allogenic hematopoietic stem-cell transplantation (allo-HSCT). The interim PMS data for 461 patients collected as of March 2021 are reported in this publication.
Methods
The case report forms (CRFs) were drafted in part by the Japanese Data Center for Hematopoietic Cell Transplantation (JDCHCT) using data elements in the Transplant Registry Unified Management Program (TRUMP) and sent to individual HSCT centers to decrease burden of reporting. These CRFs were completed by physicians in the respective HSCT centers and sent to MSD K.K., Tokyo, Japan.
Results
Allo-HSCT recipients prescribed with letermovir for CMV prophylaxis were included across 136 centers in Japan between May 2018 and March 2021. Safety and effectiveness were assessed for 460 and 373 patients, respectively. Of the patients in the safety analysis, 13.9 % experienced adverse drug reactions, the most frequent of which were renal impairment (2.2 %) and nausea (1.7 %). Among patients in the effectiveness analysis, the overall CMV antigen positivity rate was 21.2 % at Week 14 and 37.5 % at Week 24 after allo-HSCT.
Conclusions
Interim data from this largest of real-world studies confirm the safety and effectiveness of letermovir for CMV prophylaxis in Japanese allo-HSCT recipients. Given the limited data on Asian patients for letermovir use, this survey will provide valuable information for medical decision-making in routine clinical practice, serving as a vital supplement to the results obtained from clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The safety and effectiveness of the novel CMV DNA terminase inhibitor, letermovir, were investigated in a post-marketing survey involving 461 Japanese allo-HSCT patients. |
The percentage of patients with CMV antigen-positive rate was 21.2 % at Week 14 and 37.5 % at Week 24 of the survey; while the percentage of patients who were CMV antigen positive during prophylaxis with letermovir was 11.0 %. |
The percentages of patients who had any ADRs were similar to those in the Phase III letermovir trial. |
1 Introduction
Cytomegalovirus (CMV) is identified as a leading opportunistic infection observed in patients after hematopoietic stem cell transplantation (HSCT) [1]. The CMV reactivation and seropostivity of donor and/or recepient are associated with adverse prognosis after allogenic-HSCT (allo-HSCT) and increased morbidity and mortality [2,3,4]. In 2019, the estimated global CMV seroprevalence was 83 % in the general population and 86 % among blood or organ donors [5]. The CMV reactivation rate in HSCT recipients is estimated at 30–70 % [4, 6, 7].
A couple of studies have shown significant reduction in CMV reactivation with ganciclovir prophylaxis; however, improvement in survival was not observed, which can be attributed to the increase in infectious diseases due to accompanying neutropenia [8, 9]. Therefore, preemptive therapy is considered as the conventional CMV prevention strategy after HSCT [10,11,12]. Although preemptive therapy has significantly reduced the development of CMV disease subsequently reducing the mortality in HSCT recipients [6, 13, 14], CMV seropositivity and early CMV reactivation after HSCT remains associated with increased mortality [2, 15]. Therefore, there is a compelling need for a safe and effective antiviral agent for the prophylaxis of CMV in patients with allo-HSCT.
Letermovir is a CMV deoxyribonucleic acid (DNA) terminase inhibitor and its favorable safety, tolerability, and effectiveness profile displayed in a Phase III trial and real-world studies makes it a competent addition to CMV prophylaxis [16,17,18,19,20,21]. It interferes with the CMV virus terminal complex and disrupts viral replication, which is required for CMV genome packaging and virion maturation, an action mechanism distinct from other CMV agents [22]. In a global Phase III trial, letermovir was compared with placebo for CMV prophylaxis administered orally or intravenously 14 weeks after allo-HSCT in CMV-seropositive transplant recipients. Fewer patients in the letermovir group (37.5 %) presented with clinically significant CMV infection (defined as CMV disease or CMV viremia leading to preemptive treatment) compared with the placebo group (60.6 %) at Week 24 after transplantation. All-cause mortality at Week 48 after transplantation was 20.9 % in the letermovir arm and 25.5 % in the placebo arm [16]. Letermovir was approved for prophylaxis of CMV infection in adult CMV-seropositive allo-HSCT recipients by the United States Food and Drug Administration (US FDA) in 2017, and was further approved in Japan with a market authorization under orphan drug status in 2018 [23].
The safety and effectiveness of letermovir has been widely explored in real-world studies from the United States and Europe [17,18,19,20,21] but there are limited data from the Asian population. In the letermovir Phase III trial [16], there were only 58 Asian patients (40 in the letermovir arm and 18 in the placebo arm) and 36 Japanese patients (25 in the letermovir arm and 11 in the placebo arm) in spite of the number of HSCT cases in Japan (excluding autologous transplant cases) totaling 3688 in 2014 and 3724 in 2015 [24]. A recent real-world analysis published by the Japanese investigator group reported the prophylaxis data of letermovir in 114 patients [25]; however, no nationwide data on letermovir have been published to date. The post-marketing surveillance (PMS) to evaluate the safety and effectiveness of letermovir for prophylaxis of CMV infection in Japanese allo-HSCT recipients was conducted at multiple registered institutions during the survey period. Given the limited enrollment of Asian population, including Japanese, in the Phase III trial, the survey of the actual clinical use status and clinical response in Japan may contribute to the proper use of letermovir across Asia. The interim analysis results of the ongoing PMS survey are presented in this publication.
2 Methods
2.1 Survey Design
Based on the law relating to HSCT, HSCT centers in Japan are obliged to make efforts to report their HSCT outcome data to the Japanese Society for Transplantation and Cellular Therapy (JSTCT) and the Japanese Data Center for Hematopoietic Cell Transplantation (JDCHCT) using the Transplant Registry Unified Management Program (TRUMP) [26].
The JSTCT and the JDCHCT supported the transplant centers with case report forms (CRFs), which decreased the burden of reporting for the centers. PMS CRFs were drafted in part by the JDCHCT, using data reported to TRUMP, and were sent to centers. The physicians at the centers completed the PMS CRFs and sent them to MSD K.K., Tokyo, Japan.
This is an ongoing single-arm retrospective case survey with patients registered from 136 centers across Japan. This survey has an observation period of 6 months or 1 year. The registration period for patients with allo-HSCT in the current survey is planned from May 2018 to April 2022 (4 years), while the survey period is from May 2018 to April 2024 (6 years). Patients were followed up for 12 weeks, 24 weeks, and 48 weeks after transplantation. All allo-HSCT patients who received letermovir at the registered institute during the survey registration period were enrolled in the survey. Letermovir is also indicated for prophylaxis in CMV antibody-negative recipients in Japan and effectiveness in CMV antibody-negative recipients from antibody-positive donors is an effectiveness specification in the Risk Management Plan instructed by Pharmaceuticals and Medical Devices Agency (PMDA). Thus, these recipients were also included in this survey [27]. The patients were administered letermovir by accredited physicians, as part of routine care and in accordance with the local (country-specific) prescribing information at transplantation centers. Letermovir was administered via oral (tablet) or intravenous (IV) routes with/without cyclosporine (240 mg/480 mg) [28]. This observational survey used post-marketing surveillance data and was conducted in accordance with the requirements of the pharmaceutical affairs law and the ministerial ordinance of ‘Good Post-Marketing Study Practice (GPSP)’. Thus, the single-arm surveillance method was an approved condition by the Ministry of Health, Labor and Welfare.
2.2 Assessments and Outcomes
The CRFs included information on the patient’s clinical history and current status of transplantation including primary diseases, presence of CMV antibody (recipient/donor) at the time of transplant, type of human leukocyte antigen (HLA), type of donor, and presence of acute/chronic graft-versus-host disease (GVHD) after transplant. Details of therapeutic regimens received prior to transplant were also collected. The important survey items were renal impairment with IV administration, developmental and reproductive toxicity, and cardiac disorder, determined according to the risk management plan as per the PMDA in Japan. The survey primarily collected adverse drug reactions (ADRs), defined as adverse events (AEs) for which the causal relationship to letermovir could not be ruled out by the attending physician or the pharmaceutical company. The important survey items were collected regardless of relevance to the drug during the observation period. The seriousness of an AE was determined by the discretion of the company or attending physician. Effectiveness was confirmed under daily medical practice by the attending physician based on laboratory and clinical assessments: CMV disease, CMV antigen and preemptive treatment (PET). For CMV monitoring, polymerase chain reaction (PCR) or CMV antigenemia test was used at baseline, treatment initiation, Week 1, Week 8, Week 14, Week 24, and Week 48 post-transplantation. The setting of a threshold for preemptive and target therapy, as well as the treatment strategy in the event of CMV infection, was determined by the attending physician.
2.3 Statistical Analysis
For items related to safety and effectiveness, summary statistics were calculated in accordance with the nature of the data. Categorical variables, including baseline characteristics and ADRs, are presented as the count (n) and percentage (%) of patients. Continuous variables were described with standard statistics including mean or median and standard deviation (SD). All events identified as ADRs were aggregated; AEs and ADRs were defined according to the Medical Dictionary for Regulatory Activities (MedDRA) version 22.1. The Kaplan–Meier method was used to calculate patient survival and CMV antigen positivity rate. All statistical analyses were performed using Statistical Analysis System version 9.4 (SAS Institute Inc., Cary, NC, USA).
3 Results
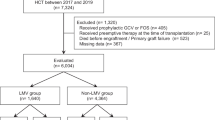
A total of 932 patients who received letermovir with allo-HSCT were registered for this survey of real-world data between May 2018 and March 2021. The CRFs were collected for 603 patients at 136 centers, whereas the CRFs for the remaining 329 patients were not collected until the development of the interim report. As of March 26, 2021, complete data were available for 461 patients from 93 centers and are included in this manuscript. Of the data procured, complete 6-month data could be recovered for 6 patients and 12-month data for the remaining 455 patients. The safety analysis was conducted for all 460 patients included in the interim analysis. The effectiveness analysis was conducted for 373 patients; of the 87 excluded patients, there were 21 patients who received letermovir for other indications and 66 patients who were not treated with the approved dosage (Fig. 1).
Patient flowchart. Letermovir indications in Japan: Prevention of cytomegalovirus (CMV) disease in allogeneic hematopoietic stem cell transplant (HSCT) patients. The usual adult dose is 480 mg as letermovir administered orally once daily. If letermovir is co-administered with cyclosporine, the dosage of letermovir is 240 mg orally once daily. Letermovir is recommended to be started between the day of allogenic HSCT and within 28 days post-transplant. The recommended duration of treatment is through 100 days post-transplant. In Japan, letermovir is also indicated for prophylaxis in CMV antibody-negative recipients
3.1 Patient Characteristics
Baseline patient characteristics for the safety analysis population are presented in Table 1. The median age in this survey was 53 (range: 4–73) years, with a higher proportion of male patients 58.0 % (n = 267) compared with female patients 42.0% (n = 193). The median duration of hospital stay at the first transplant was 86 (range: 17–472) days with 81.1 % (n = 373) of patients undergoing only 1 transplant. The prevalent primary diseases and the most important causes for transplantation were found to be acute myeloid leukemia (AML) in 40.4 % (n = 186) of patients, followed by acute lymphoblastic leukemia in 15.9 % (n = 73) of patients.
The baseline CMV serologic status included Recipient+/Donor+ (R+/D+) combination in 35.7 % (n = 164) of patients, R+/D− in 33.9 % (n = 156) of the patients, R−/D+ in 6.3 % (n = 29) of the patients, and R−/D− in 4.6 % (n = 21) of the patients. The source of stem cells for the safety analysis population was bone marrow in 35.7 % (n = 164) of patients, peripheral blood in 33.3 % (n = 153) of the patients, and cord blood in 31.1 % (n = 143) of patients. HLA matching was found in 35.7 % (n = 164) patients, while 64.1 % (n = 295) of patients reported HLA mismatching. Overall, 49.4 % (n = 227) of patients underwent myeloablative transplantation. Although GVHD prevention strategy was performed in 99.1 % (n = 456) of patients, 53.7 % (n = 247) had acute GVHD and 17.6 % (n = 81) had chronic GVHD after transplantation. A total of 95.0 % (n = 437) of the patients achieved engraftment.
The baseline characteristics for the effectiveness analysis data set are presented in Supplementary Table 1. In the effectiveness analysis population, baseline characteristics were similar to those of the safety analysis population.
In the safety analysis population, 74.4 % (n = 342) of patients received 480 mg of letermovir as their first dose without concomitant use of cyclosporine. The dosage administration pattern is presented in Table 2.
Most (71.5 %, n = 329) patients received only oral dosage form and 4.4 % (n = 20) of patients received only IV infusion, while the dosage form was switched between oral and IV in 24.1 % (n = 111) of the patients. The median duration of letermovir treatment was 84 (range: 1–521) days and the duration of oral administration (72.0, 1–238) was longer than IV administration (21.0, 1–109). The median time of first dose was 4 days after transplant (− 6 days before transplant to 100 days post-transplant). A total of 37.0 % (n = 170) of patients received first dose on Day 1 and 26.7 % (n = 123) of patients received their first dose between Days 2 and 7.
3.2 Safety
Among the 460 patients analyzed for safety, 13.9 % (n = 64) of patients had ADRs during the survey period, as presented in Table 3. The most frequent ADRs were renal impairment (2.2 %; n = 10) and nausea (1.7 %; n = 8).
ADRs were observed in 10.5 % (n = 46/440) of patients who received oral formulation and in 8.4 % (n = 11/131) who received only IV formulation. No specific ADR was more commonly observed after administration via either route (Supplementary Table 2).
AEs related to renal dysfunction and cardiac disorder, the important survey items, are shown in Table 4. Among the patients who received IV infusion of letermovir, 5.3 % (n = 7) had renal AEs. A causal relationship to letermovir could not be ruled out for 4 of these AEs: serious renal dysfunction (2 patients), serious acute kidney injury (1 patient) and non-serious increased blood creatinine (1 patient). Among patients who received an oral dose of letermovir, 4.3 % (n = 19) had renal AEs, 8 of which were ADRs.
Overall, 3.0 % of patients were reported with 14 serious cardiac AEs; a causal relationship to letermovir could not be ruled out for 3 AEs (atrial fibrillation, acute heart failure and congestive heart failure) (Table 4). The effects of drug administration in pregnant or lactating women patients or adverse reactions concerning reproductive and developmental toxicity in male patients were not reported in this survey.
The incidence of ADRs did not differ by stem cell sources; 15.9 % in bone marrow recipients, 12.4 % in peripheral blood recipients, and 13.3 % in cord blood recipients (p = 0.654). The safety by CMV antigen combination also showed no difference in the incidence of ADRs; 12.2 % in R+/D+, 12.8 % in R+/D−, 24.1 % in R−/D+, 14.3 % in R−/D− and 15.6 % in unknown group (p = 0.374).
3.3 Effectiveness
Effectiveness analysis was conducted for 80.9 % (n = 373) of patients. The percentage of patients who were CMV antigen positive was 21.2 % (n = 79) at Week 14 and 37.5 % (n = 140) at Week 24 of the survey (Fig. 2). The percentage of patients who were CMV antigen positive during prophylaxis with letermovir was 11.0 % (n = 41). Data were missing in 17 patients at both Week 14 and Week 24 (4.6 %), and patients who discontinued observation numbered 30 at Week 14 (8.0 %) and 54 at Week 24 (14.5 %). All were counted as not antigen positive.
No difference in the CMV antigen-positive rate among the stem cell sources up to Week 24; 36.9 % in bone marrow recipients, 38.7 % in peripheral blood recipients, and 38.1 % in cord blood recipients (p = 0.896). In the effectiveness by CMV antigen combination, antigen positive rate up to Week 24 tended to be higher in the recipient-positive patient group; 36.0 % in R+/D+, 47.4 % in R+/D−, 12.5 % in R−/D+ and 22.2 % in R−/D \(-\).
In Kaplan–Meier analysis, the overall CMV antigen positivity rate was 0.0 % at baseline, 22.1 % at Week 14, 42.3 % at Week 24, and 43.4 % at Week 48 (Fig. 3).
Overall, 15.0 % (n = 56) of patients received PET up to Week14 and 26.3 % (n = 98) of patients up to Week 24 (Fig. 4). The incidence of CMV PET during prophylaxis with letermovir was 6.7 % (n = 25).
There was no difference in PET rate up to Week 24 by stem cell source; 28.1 % in bone marrow recipients, 25.2 % in peripheral blood recipients and 25.4 % in cord blood recipients (p = 0.841). The rate of PET up to Week 24 also tended to be higher in the recipient-positive group; 25.6 % in R+/D+, 34.0 % in R+/D−, 8.3 % in R−/D+ and 5.6 % in R−/D−.
Onset of CMV disease was observed in 6 patients at Week 14, 11 patients at Week 24, and 12 patients at Week 48. The most frequent CMV disease was pneumonia (n = 7), followed by hepatitis (n = 2). Three of 12 patients developed CMV disease during administration of letermovir.
In Kaplan–Meier analysis, the cumulative survival rate of patients was reduced from 100 % at Week 0 to 88.6 % at Week 14, 80.6 % at Week 24 and 70.4 % at Week 48 (Fig. 5).
No mutation was reported in the drug resistance gene test.
4 Discussion
The interim results of this real-world survey demonstrated that letermovir is safe and effective in the prevention of CMV infection in Japanese patients. The interim safety and effectiveness data from the survey are consistent with the results of a Phase III clinical trial of letermovir [16] and previous real-world studies [17,18,19,20,21, 25]. The median age of the patients in the survey was 53 years, which is consistent with study patient characteristics in the letermovir arm in Phase III trial, with a median age of 53 years [16], and a previous retrospective cohort study by Johnsrud et al with a median age of 55.5 years in the letermovir arm [17]. In this survey, AML emerged as the primary reason for HSCT in 40.4 % of patients. This proportion was similar to the patient characteristics in the letermovir Phase III trial, in which 38.1 % of patients underwent HSCT due to AML [16], and the study by Johnsrud et al, in which 37.7 % patients underwent HSCT due to AML [17]. In this survey population, the percentage of HLA matching was 35.7 %, whereas more than half, that is, 64.1 % of patients, had HLA mismatch during transplantation. The percentage of HLA mismatch in this survey demonstrated consistency with the Johnsrud et al study, which showed HLA mismatch related/unrelated donor in 58.8 %, haploidentical donor in 19.3 %, and HLA matching in 25.4 % of patients [17], and a real-world study conducted in the USA that reported 56 % (n = 14) of patients with HLA mismatch, 20 % (n = 5) of patients with haploidentical and 16 % (n = 4) with HLA matching [19]. However, compared with the Phase III trial, this survey had a considerably higher proportion of cord blood as the source of stem cells (31.1 % vs 3.2 %) [16].
A total of 13.9 % of patients had at least 1 ADR in the current survey, the most common were associated with renal impairment. The percentage of patients who had renal AEs in this survey was 5.3 % after IV administration and 4.3 % after oral administration, although direct comparison is not possible due to different method or survey population, which is lower than the percentage of patients who experienced renal and urinary disorders in the letermovir group (21.7 %) of the Phase III trial [16]. In this interim survey report, 3.0 % (n = 14) of patients had AEs associated with cardiac disorder; this was lower than in the Phase III letermovir trial (12.6 %).
The CMV antigen positivity rate increased between Week 14 (22.1 %) and Week 24 (42.3 %) in the Kaplan–Meier analysis. Although the definitions of effectiveness outcomes of this survey are different from those in the Phase III trial, the trend of CMV reactivation in both studies was similar and more pronounced after Week 14 [16]. In our survey, the percentage of breakthrough CMV infection was low (11.0 % of patients were CMV-antigen positive and 6.7 % of patients had CMV preemptive antiviral treatment) while letermovir was administered. The previous Phase III clinical trial showed that while 7.4 % (24/325) of patients developed clinically significant CMV infection through Week 14 post-transplant, only 3.7 % (12/325) had breakthrough CMV infections during prophylaxis with letermovir [16]. The results of this survey showed that breakthrough CMV infection during letermovir prophylaxis is limited in the real-world setting as well as in clinical trials. The percentage of patients who were CMV-antigen positive was 21.2 % at Week 14 and 37.5 % at Week 24, and the percentage of patients who received preemptive treatment was 15.0 % at Week 14 and 26.3 % at Week 24 after transplantation. Although direct comparison is not possible because the evaluation indices are different, these percentages are comparatively higher than 17.5 % of patients with clinically significant CMV infection at Week 24 after transplantation, reported as the primary endpoint in the Phase III trial [16]. This can be attributed to the variation in CMV measurement methods in both the studies. During this survey period, CMV monitoring by polymerase chain reaction (PCR) was not reimbursed by the Japanese insurance system; hence, CMV monitoring was performed by antigenemia in Japan (antigenemia assay is reported to have a lower threshold than PCR to initiate preemptive therapy) [29]. Therefore, this survey might have observed more preemptive treatment compared with the Phase III trial. The effectiveness results of the current survey are in line with the recently published Japanese real-world analysis, which indicated that letermovir is highly effective in preventing CMV infection and reduces transplant-related mortality in allo-HSCT recipients [25]. As instructed by the local health agency, efficacy in antibody-negative recipients was also investigated in this study as part of the RMP. As expected, CMV antigen-positive and PET rates were higher in recipient-positive patients than in recipient-negative patients, but a certain number of CMV infections were observed even in R–D– cases. Although the number of recipient-negative subjects was limited in this survey, these can be important indicators for determining the intended population for letermovir. Notably, it was observed that this survey had a considerably higher proportion of patients with cord blood as the source of stem cells compared with the Phase III trial and the equivalent prophylaxis effect and ADR rate as other stem cell sources. The proportion of cord blood transplant (CBT) patients in this survey was 31.1 %, reflecting the real-world situation in Japan, where the proportion of CBT in the entire population is 23.8 % [30]. It has been reported that CBT has a high risk of CMV reactivation because of the delayed immune reconstitution [11]; however, real-world evidence of letermovir in CBT patients is limited. Recently, there have been several reports on the effects of letermovir on CBT patients [17, 18]. The study by Johnsrud et al included 26.3 % of CBT patients, with the CMV reactivation rate of 45.3 % on Day 100 in the letermovir administration group. Although the CMV reactivation rate is high in the study, it should be noted that this study included only high-risk patients for CMV reactivation including CBT patients. On the other hand, the Sharma et al study enrolled only CBT patients and had a CMV reactivation rate of 21.9 % (n = 7), with no patients requiring preemptive therapy up to Day 100 [18]. All patients in the study were initiated on prophylaxis of letermovir from Day 0 of transplantation, indicating that early treatment of letermovir for CBT patients may reduce the risk of CMV reactivation. Given this situation, the data from this survey add to the literature regarding letermovir real-world evidence in the CBT population, but further research is warranted to support these findings.
This real-world clinical practice survey based on PMS was to assess the safety and effectiveness of letermovir for prophylaxis of CMV infection in allo-HSCT recipients in Japan. However, it is acknowledged that there are some limitations to the present survey. First, as the survey included patients who received letermovir only, it had no comparator arm because, as mentioned earlier, the single arm study design was instructed by the local agency. Second, it should be noted that we do not consider competing risks in effectiveness analysis, therefore, it cannot be ruled out that the effectiveness rate may be overestimated. Third, the findings of the present survey are limited to the clinical setting in Japan. In particular, the effectiveness results in Japan, where CMV monitoring by antigenemia is the mainstay, may not be directly compared with the data from previous publications, where PCR monitoring was conducted. The direct comparison is impractical owing to the difference in the sensitivity of these two methods [31]. In addition, this survey included recipients who were negative for CMV antibodies based on Japanese indication. Therefore, the data may not be generalizable elsewhere. Finally, some participants in this survey had unknown patient characteristics. This survey included 19.6 % of patients with unknown CMV antibody combination status. However, this may be a reasonable rate given that previous studies using same data source as this survey also found that 17 % of patients’ CMV serology was not available [7]. While acknowledging these limitations, the survey provides nationwide real-world evidence for allo-HSCT recipients at high risk of CMV, from 93 different centers across Japan. In addition, to the best of the authors’ knowledge, this is the largest real-world survey in this indication with a total of 461 patients enrolled. We would like to emphasize that the importance of this survey lies in the large amount of real clinical data for Asians given limited previous data on Asian patients using letermovir. In addition, this survey is the first report on the safety and effectiveness of letermovir including the antibody-negative recipients. This can be an important reference when considering the intended population for letermovir prophylaxis. The survey is uniquely positioned and adds vital evidence for the prophylaxis of CMV in allo-HSCT patients. Further analysis of data from this survey may identify factors that influence safety and effectiveness of letermovir and assist in defining its appropriate use.
5 Conclusion
The interim data confirmed the safety and effectiveness of letermovir in allo-HSCT recipients for CMV prophylaxis among 461 patients in a real-world clinical setting in Japan. Given limited previous data on Asian patients using letermovir, the survey will provide valuable information for medical decision-making in routine clinical practice, serving as a vital supplement to the results obtained from clinical trials.
References
Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am. 2011;25(1):151–69. https://doi.org/10.1016/j.hoc.2010.11.011.
Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–38. https://doi.org/10.1182/blood-2015-11-679639.
Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122(19):3359–64. https://doi.org/10.1182/blood-2013-05-499830.
Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119–27. https://doi.org/10.1016/S2352-3026(15)00289-6.
Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019;29(3):e2034. https://doi.org/10.1002/rmv.2034.
Cho S-Y, Lee D-G, Kim H-J. Cytomegalovirus infections after hematopoietic stem cell transplantation: current status and future immunotherapy. Int J Mol Sci. 2019;20(11):2666. https://doi.org/10.3390/ijms20112666.
Takenaka K, Nishida T, Asano-Mori Y, Oshima K, Ohashi K, Mori T, et al. Cytomegalovirus Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation is Associated with a Reduced Risk of Relapse in Patients with Acute Myeloid Leukemia Who Survived to Day 100 after Transplantation: The Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol Blood Marrow Transpl. 2015;21(11):2008–16. https://doi.org/10.1016/j.bbmt.2015.07.019.
Goodrich JM, Bowden RA, Fisher L, Keller C, Schoch G, Meyers JD. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993;118(3):173–8. https://doi.org/10.7326/0003-4819-118-3-199302010-00003.
Winston DJ, Ho WG, Bartoni K, Du Mond C, Ebeling DF, Buhles WC, et al. Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients. Results of a placebo-controlled, double-blind trial. Ann Intern Med. 1993;118(3):179–84. https://doi.org/10.7326/0003-4819-118-3-199302010-00004.
Green ML, Leisenring W, Stachel D, Pergam SA, Sandmaier BM, Wald A, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2012;18(11):1687–99. https://doi.org/10.1016/j.bbmt.2012.05.015.
Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(8):260–72. https://doi.org/10.1016/S1473-3099(19)30107-0.
Einsele H, Ljungman P, Boeckh M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood. 2020;135(19):1619–29. https://doi.org/10.1182/blood.2019000956.
Goodrich JM, Mori M, Gleaves CA, Du Mond C, Cays M, Ebeling DF, et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med. 1991;325(23):1601–7. https://doi.org/10.1056/NEJM199112053252303.
Schmidt GM, Horak DA, Niland JC, Duncan SR, Forman SJ, Zaia JA. A randomized, controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants; The City of Hope-Stanford-Syntex CMV Study Group. N Engl J Med. 1991;324(15):1005–11. https://doi.org/10.1056/NEJM199104113241501.
Ljungman P, Brand R, Hoek J, de la Camara R, Cordonnier C, Einsele H, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis. 2014;59(4):473–81. https://doi.org/10.1093/cid/ciu364.
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433–44. https://doi.org/10.1056/NEJMoa1706640.
Johnsrud JJ, Nguyen IT, Domingo W, Narasimhan B, Efron B, Brown J. Letermovir prophylaxis decreases burden of cytomegalovirus (CMV) in patients at high risk for CMV disease following hematopoietic cell transplant. Biol Blood Marrow Transpl. 2020;26(10):1963–70. https://doi.org/10.1016/j.bbmt.2020.07.002.
Sharma P, Gakhar N, MacDonald J, Abidi MZ, Benamu E, Bajrovic V, et al. Letermovir prophylaxis through day 100 post transplant is safe and effective compared with alternative CMV prophylaxis strategies following adult cord blood and haploidentical cord blood transplantation. Bone Marrow Transpl. 2020;55(4):780–6. https://doi.org/10.1038/s41409-019-0730-y.
Anderson A, Raja M, Vazquez N, Morris M, Komanduri K, Camargo J. Clinical, “real-world” experience with letermovir for prevention of cytomegalovirus infection in allogeneic hematopoietic cell transplant recipients. Clin Transpl. 2020;37(7):e13866. https://doi.org/10.1111/ctr.13866.
Derigs P, Radujkovic A, Schubert ML, Schnitzler P, Schöning T, Müller-Tidow C, et al. Letermovir prophylaxis is effective in preventing cytomegalovirus reactivation after allogeneic hematopoietic cell transplantation: single-center real-world data. Ann Hematol. 2020;100(8):2087–93. https://doi.org/10.1007/s00277-020-04362-2.
Sassine J, Khawaja F, Shigle TL, Handy V, Foolad F, Aitken S, Jiang Y, Champlin R, Shpall E, Rezvani K, Ariza-Heredia EJ, Chemaly RF. Refractory and resistant cytomegalovirus after hematopoietic cell transplant in the letermovir primary prophylaxis era. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab298.
Goldner T, Hewlett G, Ettischer N, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J Virol. 2011;85(20):10884–93. https://doi.org/10.1128/JVI.05265-11.
Kim ES. Letermovir: first global approval. Drugs. 2018;78(1):147–52. https://doi.org/10.1007/s40265-017-0860-8.
JDCHCT/JSHCT. Hematopoietic cell transplantation in Japan. Annual Report of Nationwide Survey 2016. http://www.jdchct.or.jp/data/report/2016. Accessed July 2020.
Mori Y, Jinnouchi F, Takenaka K, Aoki T, Kuriyama T, Kadowaki M, et al. Efficacy of prophylactic letermovir for cytomegalovirus reactivation in hematopoietic cell transplantation: a multicenter real-world data. Bone Marrow Transpl. 2020;56(4):853–62. https://doi.org/10.1038/s41409-020-01082-z.
JSHCT. The Japan Society of Hematopoietic Cell Transplantation- Fourth guideline of Hematopoietic cell transplant. 2018. https://www.jshct.com/uploads/files/guideline/01_02_gvhd_ver04.pdf. Accessed July 2020.
PMDA. https://www.pmda.go.jp/RMP/www/170050/307fe7c6-0988-4fad-820a-1fb714aa417d/170050_6250048F1027_002RMP.pdf. Accessed July 2020.
Merck & Co., Inc., Kenilworth, NJ, USA. PREVYMIS™ (letermovir) tablets, for oral use: prescribing information 2021. Available from: https://www.merck.com/product/usa/pi_circulars/p/prevymis/prevymis_pi.pdf. Accessed July 2020.
Kanda Y, Yamashita T, Mori T, Ito T, Tajika K, Mori S, et al. A randomized controlled trial of plasma real-time PCR and antigenemia assay for monitoring CMV infection after unrelated BMT. Bone Marrow Transpl. 2010;45(8):1325–32. https://doi.org/10.1038/bmt.2009.337.
JDCHCT/JSHCT. Hematopoietic cell transplantation in Japan. Annual Report of Nationwide Survey 2019. http://www.jdchct.or.jp/data/report/2019. Accessed July 2020.
Yakushiji K, Gondo H, Kamezaki K, Shigematsu K, Hayashi S, Kuroiwa M, et al. Monitoring of cytomegalovirus reactivation after allogeneic stem cell transplantation: comparison of an antigenemia assay and quantitative real-time polymerase chain reaction. Bone Marrow Transpl. 2002;29(7):599–606. https://doi.org/10.1038/sj.bmt.1703513.
Acknowledgements
We thank the JSTCT and the JDCHCT for supporting the transplant centers with the CRFs which decreased the burden of reporting. The authors would like to thank Vibha Dhamija and Yukti Singh from IQVIA for their medical writing and editing support and CMIC HOLDINGS Co., Ltd for technical assistance with data management and statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This article was funded by MSD K.K., Tokyo, Japan. The authors had full control of the content, and approved the final version
Conflict of interest
Itaru Hiraishi, Rie Ueno, Asuka Watanabe, and Shinichiroh Maekawa are employees of MSD K.K., Tokyo, Japan which sponsored the research and manuscript development. The authors have no other conflicts of interest to declare.
Availability of Data and Material
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA’s data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the study data can be submitted through the Engage Zone site or via email to dataaccess@merck.com.
Code Availability
Not applicable.
Author contributions
All authors participated in the writing, editing, and critical revision for intellectual content, and approval of the final version of this manuscript. All authors met ICMJE authorship criteria and agree to be accountable for all aspects of the work.
Ethics Approval
This survey was carried out in accordance with Good Post-Marketing Study Practice for Drugs as specified by the Ministry of Health, Labor and Welfare in Japan. A research protocol was created for the survey and was approved by an external Institution Review Board.
Consent to Participate
According to Good Post-Marketing Study Practice in Japan, informed consent was not required for this post-marketing survey. As such, informed consents were voluntarily obtained from the individual participants in some facilities and all facilities agreed to use the survey data by a contract or an agreement form with MSD K.K., Tokyo, Japan.
Consent for Publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hiraishi, I., Ueno, R., Watanabe, A. et al. Safety and Effectiveness of Letermovir in Allogenic Hematopoietic Stem Cell Transplantation Recipients: Interim Report of Post-marketing Surveillance in Japan. Clin Drug Investig 41, 1075–1086 (2021). https://doi.org/10.1007/s40261-021-01096-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01096-5