Abstract
Background
Migraine is a common, chronic neurovascular brain disorder with non-negligible multifaceted economic costs. Existing preventive treatments involve the selective use of onabotulinumtoxinA, which aims at migraine morbidity reduction for patients who have failed initial preventive treatment with oral agents. Erenumab is a new preventive treatment for migraines.
Objective
To evaluate the differences in costs and outcomes of the preventive treatment with erenumab versus onabotulinumtoxinA in patients with chronic migraines (CM) in Greece to assess the economic value of this treatment.
Methods
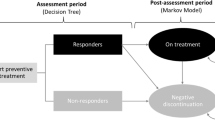
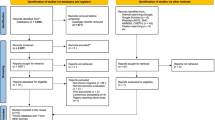
We conducted a cost-effectiveness analysis from both the payer and the societal perspective using a decision-tree analytic model. Outcomes were expressed in migraines avoided and in quality-adjusted life-years (QALYs). We obtained model inputs from the existing literature. The decision path adjusted for variation in the probability of adherence and the resulting differential effectiveness between the two treatments. Direct costs included the cost of the two drugs and administration costs, the costs of acute drugs used under usual care, and the costs of hospitalization, physician, and emergency department visits. Indirect costs for the societal perspective analyses included wages lost on workdays. The time-horizon of the analysis was 1 year and all costs were calculated in 2019 euros (€). Sensitivity analyses were conducted to control for parameter uncertainty and to evaluate the robustness of the findings.
Results
Our results indicate that treatment of CM with erenumab compared to onabotulinumtoxinA resulted in incremental cost-effectiveness ratios (ICERs) of €218,870 and €231,554 per QALY gained and €620 and €656 per migraine avoided, from the societal and the payer’s perspective, respectively. Using a common cost-effectiveness threshold equal to three times the local gross domestic product (GDP) per capita (€49,000), for the erenumab ICERs to fall below this threshold, the erenumab price would have to be no more than €192 (societal perspective) or €173 (payer perspective).
Conclusion
The prophylactic treatment of CM with erenumab in Greece might be cost effective compared to the existing alternative of onabotulinumtoxinA from both the payer and the societal perspective, but only at a highly discounted price. Nevertheless, erenumab could be considered a therapeutic option for patients who fail treatment with onabotulinumtoxinA.



Similar content being viewed by others
References
Leonardo M, Steiner T, Scher A. The global burden of migraine: Measuring disability in headache disorders with WHO’s Classification of Functioning, Disability and Health (ICF). J Headache Pain. 2005;6:429–40.
Lipton RB, Stewart W, Diamond S, Diamond M, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–57.
Brandes JL. Global trends in migraine care: results from the MAZE survey. CNS Drugs. 2002;16:13–8.
Mitsikostas D-D, Arvaniti C, Constantidis T, et al. A population based survey for headaches in Greece to estimate prevalence and treatment preferences. Neurology. 2018;90(P3):143.
Munakata J, Wang S, Rupnow M. Economic burden of transformed migraine: Results for the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2008;48:553–63.
Messali A, Sanderson J, Blumenfeld AM. Direct and indirect costs of chronic and episodic migraine in the United States: a web-based survey. Headache. 2016;56:306–22.
Serrano D, Manack AN, Reed ML, Buse DC, Varon SF, Lipton RB. Cost and predictors of lost productive time in chronic migraine and episodic migraine : results from the American Migraine Prevalence and Prevention (AMPP) Study. Value Health. 2013;16(1):31–8.
Bonafede M, Sapra S, Shah N. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache. 2018;58:700–15.
D’Amico D, Grazzi L, Moschiano F, Bussone G. Topiramate in migraine prophylaxis. Neurol Sci. 2005;26:s130–3.
Snow V, Weiss K, Wall E, Mottur-Pilson C. Pharmacologic management of acute attacks of migraine and prevention of migraine headache. Ann Intern Med. 2002;137:840–9.
Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, Sándor PS. EFNS guideline on the drug treatment of migraine–revised report of an EFNS task force. Eur J Neurol. 2009;16(9):968–81.
Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754–63.
Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3–20.
Silberstein SD, Holland S, Freitag F. Evidence-based guideline update: Pharmacologic treatment for episodic migraine prevention in adults: report of the quality standards subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337–45.
Bagnato F, Good J. The use of antiepileptics in migraine prophylaxis. Headache. 2016;3:603–15.
Yu J, Smith KJ, Brixner DI. Cost effectiveness of pharmacotherapy for the prevention of migraine. CNS Drugs. 2010;24(8):695–712.
Diener HC, Dodick DW, Aurora SK, Turkel CC. OnabotulinumtoxinA for treatment of chronic migraine: results from placebo-controlled phase of the PREEMPT 2 trial. Cephalagia. 2010;30(7):804–14.
Diener HC, Dodick DW, Turkel CC, Demos G, Degryse RE, Earl NL, Brin MF. Pooled analysis of the safety and tolerability of onabotulinumtoxinA in the treatment of chronic migraine. Eur J Neurol. 2014;21(6):851–9.
Dodick DW, Turkel CC, Degryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT Clinical Program. Headache. 2010;50(6):921–36.
Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary : botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache Academy of Neurology. Neurology. 2016;86(19):1818–26.
Brown JS, Papadopoulos G, Neumann PJ, Price M, Friedman M, Menzin J. Cost-effectiveness of migraine prevention : the case of topiramate in the UK. Cephalalgia. 2006;26(12):1473–82.
Silberstein SD, Lipton RB, Dodick D, et al. Efficacy and safety of topiramate for the treatment of chronic migraine: a randomized, double-blind, placebo-controlled trial. Headache. 2007;47(2):170–80.
Brandes JL, Saper JR, Diamond M, et al. Topiramate for Migraine prevention. JAMA. 2004;291(8):965–73.
Láinez MJ. The effect of migraine prophylaxis on migraine-related resource use and productivity. CNS Drugs. 2009;23(9):727–38.
Silberstein S, Lipton R, Dodick D, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache. 2009;49(8):1153–62.
Batty AJ, Hansen RN, Bloudek LM, et al. The cost-effectiveness of onabotulinumtoxinA for the prophylaxis of headache in adults with chronic migraine in the UK. J Med Econ. 2013;16(7):877–87.
Vikelis M, Argyriou AA, Dermitzakis EV, Spingos KC, Mitsikostas DD. Onabotulinumtoxin-A treatment in Greek patients with chronic migraine. J Headache Pain. 2016;17(1):84.
Lattanzi S, Brigo F, Trinka E, Vernieri F, Corradetti T, Dobran M, Silvestrini M. Erenumab for preventive treatment of migraine: a systematic review and meta-analysis of efficacy and safety. Drugs. 2019;79(4):417–31.
Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338–50.
FDA Approves Aimovig (erenumab-aooe), a novel treatment developed specifically for migraine prevention. Amgen. https://www.amgen.com/media/newsreleases/2018/05/fda-approves-aimovig-erenumabaooe-a-novel-treatment-developed-specifically-for-migraine-prevention/. Accessed Nov 2018.
https://www.ema.europa.eu/en/medicines/human/EPAR/aimovig. Accessed Nov 2018.
Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine : a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425–34.
Lipton RB, Tepper SJ, Reuter U, Silberstein S, Stewart WF, Nilsen J, Leonardi DK, Desai P, Cheng S, Mikol DD, Lenz R. Erenumab in chronic migraine: Patient-reported outcomes in a randomized double-blind study. Neurology. 2019;92(19):e2250–60.
Sussman M, Benner J, Neumann P, Menzin J. Cost-effectiveness analysis of erenumab for the preventive treatment of episodic and chronic migraine: results from the US societal and payer perspectives. Cephalalgia. 2018;38(10):1644–57.
Brown JS, Papadopoulos G, Neumann PJ, Friedman M, Miller JD, Menzin J. Cost-effectiveness of topiramate in migraine prevention : results from a pharmacoeconomic model of topiramate treatment. Headache. 2005;45(8):1012–22.
Landy SH, Runken MC, Bell CF, Higbie RL, Haskins LS. Assessing the impact of migraine onset on work productivity. J Occup Environ Med. 2011;53(1):74–81.
Lipton RB, Brennan A, Palmer S, Hatswell AJ, Porter JK, Sapra S, Villa G, Shah N, Tepper S, Dodick D. Estimating the clinical effectiveness and value-based price range of erenumab for the prevention of migraine in patients with prior treatment failures: a US societal perspective. J Med Econ. 2018;21(7):666–75.
Caro G, Getsios D, Caro J. Sumatriptan: economic evidence for its use in the treatment of migraine, the Canadian economic analysis. Cephalalgia. 2001;21:12–9.
Caro J, Caro G, Getsios D. The migraine ACE model: evaluating the impact on time lost and medical resource use. Headache. 2000;42:282–91.
https://www.galinos.gr. Accessed Feb 2019.
Foundation for Economic and Industrial Research (IOBE). Rationalization of Pharmaceutical Pricing System in Greece. Athens: Foundation for Economic and Industrial Research (IOBE) (2013).
https://www.sfee.gr/fek-1702v1-8-2011-klista-enopiimena-nosilia-ke-imerisio-nosilio-sto-esi/. Accessed Nov 2018.
Institute for Clinical and Economic Review (ICER). Calcitonin Gene-Related Peptide (CGRP) Inhibitors as Preventive Treatments for Patients with Episodic or Chronic Migraines: Effectiveness and Value. Final Evidence Report. July 3, 2018.
Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost–effectiveness of interventions: alternative approaches. Bull World Health Organ. 2014;93:118–24.
Fendrick AM, Smith DG, Chernew ME, Shah SN. A benefit-based copay for prescription drugs: patient contribution based on total benefits, not drug acquisition cost. Am J Manag Care. 2001;7(9):861–74.
Giannouchos T. Comparative Analysis of Pharmaceutical Expenditure in Greece and the countries of the Eurozone the years 2005–2016 (in Greek). 2016. http://dione.lib.unipi.gr/xmlui/bitstream/handle/unipi/10259/Giannouxos_Theodoros.pdf?sequence=1&isAllowed=y. Accessed Dec 2018.
Campbell EG, Gruen RL, Mountford J, Miller LG, Cleary PD, Blumenthal D. A national survey of physician-industry relationships. N Engl J Med. 2007;356(17):1742–50.
Stevenson FA, Greenfield SM, Jones M, Nayak A, Bradley CP. GPs’ perceptions of patient influence on prescribing. Fam Pract. 1999;16(3):255–61.
Horodnic AV, Williams CC, Polese A, Zait A, Oprea L. Exploring the practice of making informal payments in the health sector: some lessons from Greece. In: The informal economy in global perspective 2017. Cham: Palgrave Macmillan; 2017. p. 157–72.
Lewis M. Informal payments and the financing of health care in developing and transition countries. Health Aff. 2007;26(4):984–97.
Charles C, Whelan T, Gafni A. What do we mean by partnership in making decisions about treatment? BMJ. 1999;319(7212):780–2.
Mathew PG, Pavlovic JM, Lettich A, Wells RE, Robertson CE, Mullin K, Charleston L IV, Dodick DW, Schwedt TJ. Education and decision making at the time of Triptan prescribing: patient expectations vs actual practice. Headache. 2014;54(4):698–708.
Payne KA, Varon SF, Kawata AK, Yeomans K, Wilcox TK, Manack A, Buse DC, Lipton RB, Goadsby PJ, Blumenfeld AM. The International Burden of Migraine Study (IBMS): study design, methodology, and baseline cohort characteristics. Cephalalgia. 2011;31(10):1116–30.
Shiroiwa T, Fukuda T, Ikeda S, Shimozuma K. QALY and productivity loss: empirical evidence for “double counting”. Value Health. 2013;16(4):581–7.
Acknowledgements
The authors would like to acknowledge Mr. Aristeidis Draganigos for his valuable input into the methodological part of this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the coordination of the study and drafting of the manuscript. TVG conceived and designed the study. TVG led the model development and performed the analyses. RLO and DDM contributed to the design and development of the model. DDM provided overall direction to the study. RLO, AV, and PK critically reviewed the analyses and assisted with the interpretation of the results. All authors reviewed and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Ethical standards
This study used data from existing published sources in a computational model. The research did not entail the use of data for any human subject at an individual level; thus institutional review board approval and informed consent were deemed unnecessary.
Funding
The authors did not receive any funding for this study.
Conflict of interest
The authors declare that they have no conflicts of interest. Other disclosures: DDM received honoraria for lecturing, consulting fees or research grants form Sanofi-Genzyme, Amgen, Allergan, Cefaly, Electrocore, Eli Lilly, Novartis, Merz, Merck-Serono, Roche, and Teva and has participated in clinical trials for agents analyzed in this study, which were however unrelated to this analysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Giannouchos, T.V., Mitsikostas, DD., Ohsfeldt, R.L. et al. Cost-Effectiveness Analysis of Erenumab Versus OnabotulinumtoxinA for Patients with Chronic Migraine Attacks in Greece. Clin Drug Investig 39, 979–990 (2019). https://doi.org/10.1007/s40261-019-00827-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00827-z