Abstract
Background and Objectives
Giving the human papillomavirus (HPV) vaccination to females has been shown to be cost-effective in most countries. The epidemiological evidence and economic burden of HPV-related diseases have gradually been shown to be gender neutral. Randomized clinical trials report high efficacy, immunogenicity and safety of the HPV vaccine in males aged 16–26 years. Some pioneering countries extended their HPV vaccination programme to include males, regardless of the cost-effectiveness analysis results. Nevertheless, decision makers need evidence provided by modelling and economic studies to justify the funding of mass vaccination. This systematic review aims to assess the cost-effectiveness of extending the HPV vaccination programme to include males living in high-income countries.
Methods
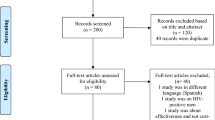
A systematic review of the cost-effectiveness analyses of HPV vaccination in males was performed. Data were extracted and analysed using a checklist adapted from the Consolidated Health Economic Evaluation Reporting Standards Statement.
Results
Seventeen studies and 12 underlying mathematical models were identified. Model filiation showed evolution in time from aggregate models (static and dynamic) to individual-based models. When considering the health outcomes HPV vaccines are licensed for, regardless of modelling approaches and assumptions, extending vaccinations to males is rarely found to be cost-effective in heterosexual populations. Cost-effectiveness ratios become more attractive when all HPV-related diseases are considered and when vaccine coverage in females is below 40 %.
Conclusion
Targeted vaccination of men who have sex with men (MSM) seems to be the best cost-effectiveness option. The feasibility of this strategy is still an open question, since early identification of this specific population remains difficult.



Similar content being viewed by others
References
Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J. Clin. Virol. 2005;32(Supplement):16–24.
Schiffman M, Kjaer SK. Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003;31:14–9.
IARC Working Group on the Evaluation of Carcinogenic. Risks to Humans. Human papillomaviruses. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2007;90:1–636.
Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23.
De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int. J. Cancer. 2009;124:1626–36.
Gillison M. HPV and its effect on head and neck cancer prognosis. Clin. Adv. Hematol. Oncol. 2010;8:680–2.
Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;26(Suppl 10):K17–28.
Harper DM. Currently approved prophylactic HPV vaccines. Expert Rev. Vaccines. 2009;8:1663–79.
IARC Working Group. Primary end-points for prophylactic HPV vaccine trials. Geneva: World Health Organisation; 2013 Sep.
Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Supplement 5):F123–38.
Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nat. Rev. Clin. Oncol. 2013;10:400–10.
Jit M, Brisson M. Modelling the epidemiology of infectious diseases for decision analysis: a primer. PharmacoEconomics. 2011;29:371–86.
Canfell K, Chesson H, Kulasingam SL, Berkhof J, Diaz M, Kim JJ. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine. 2012;30(Suppl 5):F157–67.
Markowitz LE, Tsu V, Deeks SL, Cubie H, Wang SA, Vicari AS, et al. Human papillomavirus vaccine introduction—the first five years. Vaccine. 2012;30(Suppl 5):F139–48.
Lehtinen M, Paavonen J. Impact of human papillomavirus vaccination depends on effective vaccination strategy. Int. J. Cancer. 2009;125:1490–1.
Stanley M. Perspective: vaccinate boys too. Nature. 2012;488:S10.
Chesson HW, Ekwueme DU, Saraiya M, Watson M, Lowy DR, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30:6016–9.
Ali H, Donovan B, Wand H, Read TRH, Regan DG, Grulich AE, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ. 2013;346:f2032–f2032.
Kreuter A, Wieland U. Human papillomavirus-associated diseases in HIV-infected men who have sex with men. Curr. Opin. Infect. Dis. 2009;22:109–14.
Palefsky JM. Human papillomavirus-related disease in men: not just a women’s issue. J. Adolesc. Health. 2010;46:S12–9.
Hillman RJ, Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Vardas E, et al. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin. Vaccine Immunol. 2012;19:261–7.
Luyten J, Engelen B, Beutels P. The sexual ethics of HPV vaccination for boys. HEC Forum. 2014;26:27–42.
Marty R, Roze S, Bresse X, Largeron N, Smith-Palmer J. Estimating the clinical benefits of vaccinating boys and girls against HPV-related diseases in Europe. BMC Cancer. 2013;13:10.
Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), MMWR Morb. Mortal. Wkly. Rep. 2011;2011(60):1705–8.
Wilkinson E. Australia leads way on HPV vaccination in boys. Lancet Infect. Dis. 2012;12:831–2.
Quinn S, Goldman RD. Human papillomavirus vaccination for boys. Can. Fam. Physician. 2015;61:43–6.
Impfkommission S (STIKO). Empfehlung der sächsischen Impfkommission zur Durchführung von Schutzimpfungen im Freistaat Sachsen. 2014. http://www.gesunde.sachsen.de/download/lua/LUA_HM_Impfempfehlungen_E1.pdf
Seto K, Marra F, Raymakers A, Marra CA. The cost effectiveness of human papillomavirus vaccines: a systematic review. Drugs. 2012;72:715–43.
Jiang Y, Gauthier A, Postma MJ, Ribassin-Majed L, Largeron N, Bresse X. A critical review of cost-effectiveness analyses of vaccinating males against human papillomavirus. Hum. Vaccines Immunother. 2013;9:2285–95.
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 2010;8:336–41.
Drummond MF, Richardson WS, O’Brien BJ, Levine M, Heyland D. Users’ guides to the medical literature. XIII. How to use an article on economic analysis of clinical practice. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1997;277:1552–7.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–50.
Shi L, Hodges M, Drummond M, Ahn J, Li SC, Hu S, et al. Good research practices for measuring drug costs in cost-effectiveness analyses: an international perspective: the ISPOR Drug Cost Task Force report—part VI. Value Health. 2010;13:28–33.
National Board of Health, Danish Centre for Health Technology Assessment. Reduction in the risk of cervical cancer by vaccination against human papillomavirus (HPV)—a health technology assessment. Copenhagen: National Board of Health, Danish Centre for Health Technology Assessment; 2007.
Elbasha EH, Dasbach EJ, Insinga RP. A multi-type HPV transmission model. Bull. Math. Biol. 2008;70:2126–76.
Comité sur l’immunisation du Québec, HPV Ad Hoc Scientific Committee. HPV vaccination in Québec: knowledge update and expert panel proposals [Internet]. Institut national de santé publique du Québec; Available from: http://www.santecom.qc.ca/Bibliothequevirtuelle/INSPQ/9782550687573.pdf
Olsen J, Jepsen MR. Human papillomavirus transmission and cost-effectiveness of introducing quadrivalent HPV vaccination in Denmark. Int. J. Technol. Assess. Health Care. 2010;26:183–91.
Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg. Infect. Dis. 2007;13:28–41.
Laprise J-F, Drolet M, Boily M-C, Jit M, Sauvageau C, Franco EL, et al. Comparing the cost-effectiveness of two- and three-dose schedules of human papillomavirus vaccination: a transmission-dynamic modelling study. Vaccine. 2014;32:5845–53.
Kulasingam S, Connelly L, Conway E, Hocking JS, Myers E, Regan DG, et al. A cost-effectiveness analysis of adding a human papillomavirus vaccine to the Australian National Cervical Cancer Screening Program. Sex. Health. 2007;4:165–75.
Kulasingam SL, Myers ER. Potential health and economic impact of adding a human papillomavirus vaccine to screening programs. JAMA. 2003;290:781–9.
Myers ER, Green S, Lipkus I. Patient preferences for health states related to HPV infection: visual analogue scales versus time trade-off elicitation. Proceedings of the 21st International Papillomavirus Conference; Mexico City; 2004.
Regan DG, Philp DJ, Hocking JS, Law MG. Modelling the population-level impact of vaccination on the transmission of human papillomavirus type 16 in Australia. Sex. Health. 2007;4:147–63.
Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg. Infect. Dis. 2004;10:1915–23.
Sanders GD, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg. Infect. Dis. 2003;9:37–48.
Myers ER, McCrory DC, Nanda K, Bastian L, Matchar DB. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am. J. Epidemiol. 2000;151:1158–71.
Zechmeister I, de Blasio BF, Garnett G, Neilson AR, Siebert U. Cost-effectiveness analysis of human papillomavirus-vaccination programs to prevent cervical cancer in Austria. Vaccine. 2009;27:5133–41.
Neilson A, Freiesleben de Blasio B. Economic evaluation of the human papillomavirus (HPV)-vaccination in Norway. Oslo: Norwegian Knowledge Centre for the Health Services; 2007.
Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine. 2006;24 Suppl 3:S3/178–86.
Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ. 2008;337:a769.
Choi YH, Jit M, Gay N, Cox A, Garnett G, Edmunds WJ. Developing a model of the transmission of HPV and development of HPV-related diseases. 24th International Papillomavirus Conference Clinical Workshop; Beijing; 2007.
Choi YH, Jit M, Gay N, Cox A, Garnett GP, Edmunds WJ. Transmission dynamic modelling of the impact of human papillomavirus vaccination in the United Kingdom. Vaccine. 2010;28:4091–102.
Usher C, Tilson L, Olsen J, Jepsen M, Walsh C, Barry M. Cost-effectiveness of human papillomavirus vaccine in reducing the risk of cervical cancer in Ireland due to HPV types 16 and 18 using a transmission dynamic model. Vaccine. 2008;26:5654–61.
Pearson AL, Kvizhinadze G, Wilson N, Smith M, Canfell K, Blakely T. Is expanding HPV vaccination programs to include school-aged boys likely to be value-for-money: a cost-utility analysis in a country with an existing school-girl program. BMC Infect. Dis. 2014;14:351.
Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28:6858–67.
Burger EA, Sy S, Nygård M, Kristiansen IS, Kim JJ. Prevention of HPV-related cancers in Norway: cost-effectiveness of expanding the HPV vaccination program to include pre-adolescent boys. PloS One. 2014;9:e89974.
Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29:8443–50.
Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ. 2009;339:b3884.
Blakely T, Kvizhinadze G, Karvonen T, Pearson AL, Smith M, Wilson N. Cost-effectiveness and equity impacts of three HPV vaccination programmes for school-aged girls in New Zealand. Vaccine. 2014;32:2645–56.
Brisson M, van de Velde N, Franco EL, Drolet M, Boily M-C. Incremental impact of adding boys to current human papillomavirus vaccination programs: role of herd immunity. J. Infect. Dis. 2011;204:372–6.
Chesson HW, Ekwueme DU, Saraiya M, Markowitz LE. Cost-effectiveness of human papillomavirus vaccination in the United States. Emerg. Infect. Dis. 2008;14:244–51.
Goldhaber-Fiebert JD, Stout NK, Ortendahl J, Kuntz KM, Goldie SJ, Salomon JA. Modeling human papillomavirus and cervical cancer in the United States for analyses of screening and vaccination. Popul. Health Metr. 2007;5:11.
Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N. Engl. J. Med. 2008;359:821–32.
Kim JJ, Kuntz KM, Stout NK, Mahmud S, Villa LL, Franco EL, et al. Multiparameter calibration of a natural history model of cervical cancer. Am. J. Epidemiol. 2007;166:137–50.
Kim JJ, Andres-Beck B, Goldie SJ. The value of including boys in an HPV vaccination programme: a cost-effectiveness analysis in a low-resource setting. Br. J. Cancer. 2007;97:1322–8.
Goldie SJ, Kim JJ, Kobus K, Goldhaber-Fiebert JD, Salomon J, O’shea MKH, et al. Cost-effectiveness of HPV 16, 18 vaccination in Brazil. Vaccine. 2007;25:6257–70.
Van de Velde N, Brisson M, Boily M-C. Understanding differences in predictions of HPV vaccine effectiveness: a comparative model-based analysis. Vaccine. 2010;28:5473–84.
Van de Velde N, Boily M-C, Drolet M, Franco EL, Mayrand M-H, Kliewer EV, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J. Natl. Cancer Inst. 2012;104:1712–23.
Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect. Dis. 2010;10:845–52.
Deshmukh AA, Chiao EY, Das P, Cantor SB. Clinical effectiveness and cost-effectiveness of quadrivalent human papillomavirus vaccination in HIV-negative men who have sex with men to prevent recurrent high-grade anal intraepithelial neoplasia. Vaccine. 2014;32:6941–7.
Marra F, Cloutier K, Oteng B, Marra C, Ogilvie G. Effectiveness and cost effectiveness of human papillomavirus vaccine: a systematic review. PharmacoEconomics. 2009;27:127–47.
Jit M, Brisson M, Laprise J-F, Choi YH. Comparison of two dose and three dose human papillomavirus vaccine schedules: cost effectiveness analysis based on transmission model. BMJ. 2015;350:g7584.
Postma MJ, Jit M, Rozenbaum MH, Standaert B, Tu H-A, Hutubessy RC. Comparative review of three cost-effectiveness models for rotavirus vaccines in national immunization programs; a generic approach applied to various regions in the world. BMC Med. 2011;9:84.
Olsen J, Jørgensen TR. Revisiting the cost-effectiveness of universal HPV-vaccination in Denmark accounting for all potentially vaccine preventable HPV-related diseases in males and females. Cost Eff. Resour. Alloc. 2015;13:4.
Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum. Vaccines Immunother. 2014;10:1155–65.
Bogaards JA, Kretzschmar M, Xiridou M, Meijer CJLM, Berkhof J, Wallinga J. Sex-specific immunization for sexually transmitted infections such as human papillomavirus: insights from mathematical models. PLoS Med. 2011;8:e1001147.
Smith MA, Canfell K. Incremental benefits of male HPV vaccination: accounting for inequality in population uptake. PLoS ONE. 2014;9:e101048.
Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015;372:711–23.
US Food and Drug Administration. FDA approves Gardasil 9 for prevention of certain cancers caused by five additional types of HPV [Internet]. 2014 [cited 2015 Mar 27]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm426485.htm
Petrosky E, Bocchini JA, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2015;64:300–4.
Acknowledgments
We thank Dr Donna Willis, MD, MPH, Johns Hopkins Medical School, Baltimore, USA, for her help in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used in the preparation of this manuscript.
Conflict of Interest
Mohamed-Béchir Ben Hadj Yahia, Anaïs Jouin-Bortolotti and Benoît Dervaux have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ben Hadj Yahia, MB., Jouin-Bortolotti, A. & Dervaux, B. Extending the Human Papillomavirus Vaccination Programme to Include Males in High-Income Countries: A Systematic Review of the Cost-Effectiveness Studies. Clin Drug Investig 35, 471–485 (2015). https://doi.org/10.1007/s40261-015-0308-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0308-4