Abstract
Background and Objectives
The aim of this study was to evaluate the effectiveness and safety of sublingual fentanyl oral disintegrating tablets (sublingual fentanyl ODT) for the treatment of breakthrough pain (BTP), cancer or non-cancer related, in terms of relief of pain intensity, adverse events (AEs) and patient satisfaction, and to further examine the clinical and epidemiological profile of patients with BTP in a clinical setting.
Methods
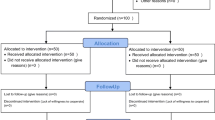
A multicentre, prospective, open-label study was conducted in 19 pain units from Catalonia hospitals (Spain) over a 1-month period. Opioid-tolerant adult patients experiencing episodes of BTP intensity >5 on a visual analogue scale (VAS) during the 12–24 h before screening or AEs related to their previous rescue medication for BTP received sublingual fentanyl ODT in the course of routine clinical practice and completed a 30-day study period consisting of five assessment points: days 0 (baseline), 3, 7, 15 and 30. The efficacy was assessed by collecting pain intensity and pain relief data at baseline and at each assessment. AEs were recorded by investigators throughout the study during clinic visits and telephone follow-ups. For all patients, titration was begun with an initial dose of 100 μg. No more than two doses were allowed to treat an episode and patients might wait at least 4 h before treating another BTP episode with sublingual fentanyl ODT. The dose was increased by 100 μg multiples up to 400 μg as needed; and by 200 μg multiples up from 400 to 800 μg, the maximum titration step.
Results
A total of 182 patients were enrolled and 177 (97.2 %) completed the study: 37 had breakthrough cancer pain (BTcP) and 145 had breakthrough non-cancer pain (BTncP). The mean pain intensity showed a statistically significant improvement at the first assessment point and at all assessments thereafter (p < 0.0001). At the end of the study, the time lag between administration and first effect of sublingual fentanyl ODT was ≤10 min in 69.0 % (60 % BTcP and 71.2 % BTncP). The number of daily BTP episodes decreased in both groups, but it was statistically significant in BTcP. 114 patients (62.64 %) experienced AEs during the study. AEs recorded included nausea, vomiting, somnolence and constipation, and seven (4.49 %) were considered severe. No death or discontinuation was considered related to AEs.
Conclusion
Sublingual fentanyl ODT provided rapid and consistent relief from BTP, both in cancer and non-cancer patients. It was well-tolerated and well-accepted by patients in routine clinical practice.





Similar content being viewed by others
References
Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273–81.
Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81:129–34.
Caraceni A, Martini C, Zecca E, et al. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliat Med. 2004;18:177–83.
Mercadante S, Maddaloni S, Roccella S, et al. Predictive factors in advanced cancer pain treated only by analgesics. Pain. 1992;50:151–5.
Bruera E, Schoeller T, Wenk R, et al. A prospective multicenter assessment of the Edmonton staging system for cancer pain. J Pain Symptom Manag. 1995;10:348–55.
Portenoy R, Bruns D, Shoemaker B, et al. Breakthrough pain in community-dwelling patient with cancer pain and non-cancer pain. Part 1: prevalence and characteristics. J Opioid Manag. 2010;6:97–108.
Manchikanti L, Singh V, Caraway DL, et al. Breakthrough pain in chronic non-cancer pain: fact, fiction or abuse. Pain Physician. 2011;14:E103–17.
Fine PG, Narayana A, Passik SD. Treatment of breakthrough pain with fentanyl buccal tablet in opioid-tolerant patients with chronic pain: appropriate patient selection and management. Pain Med. 2010;11:1024–36.
Passik SD. Issues in long-term opioid therapy: unmet needs, risks, and solutions. Mayo Clin Proc. 2009;84:593–601.
Portenoy RK, Bennett DS, Rauck R, et al. Prevalence and characteristics of breakthrough pain in opioid-treated patients with chronic non-cancer pain. J Pain. 2006;7:583–91.
Zeppetella G. Opioids for cancer breakthrough pain: a pilot study reporting patients’ assessment of time to meaningful pain relief. J Pain Symptom Manag. 2008;35:563–7.
De Sanctis Briggs V. Opioid therapy in cancer patients. Dolor. 2011;26:29–36.
Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci. 1998;1:15–30.
Brendenberg S, Duberg M, Lennernäs B, et al. In vitro and in vivo evaluation of a new sublingual tablet system for rapid oromucosal absorption using fentanyl citrate as the active substance. Eur J Pharm Sci. 2003;20:327–34.
Abstral® (fentanyl sublingual tablets for breakthrough cancer pain). PT 2011; 36:2–28.
Rauck RL, Tark M, Reyes E, et al. Efficacy and long-term tolerability of sublingual fentanyl orally disintegrating tablet in the treatment of breakthrough cancer pain. Curr Med Res Opin. 2009;25:2877–85.
Lennernäs B, Frank-Lissbrant I, Lennernäs H, et al. Sublingual administration of fentanyl to cancer patients is an effective treatment for breakthrough pain: results from a randomized phase II study. Palliat Med. 2010;24:286–93.
Nalamachu S, Hassman D, Wallace MS, et al. Long-term effectiveness and tolerability of sublingual fentanyl orally disintegrating tablet for the treatment of breakthrough cancer pain. Curr Med Res Opin. 2011;27:519–30.
Darwish M, Xie F. Pharmacokinetics of fentanyl buccal tablet: a pooled analysis and review. Pain Pract. 2012;12:307–14.
Portenoy RK, Messina J, Xie F. Fentanyl buccal tablet for the treatment of breakthrough pain in opioid-treated patients with chronic low back pain: a randomized, placebo-controlled study. Curr Med Res Opin. 2007;23:223–33.
Simpson DM, Messina J, Xie F, et al. Fentanyl buccal tablet for the relief of breakthrough pain in opioid-tolerant adult patients with chronic neuropathic pain: a multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29:588–601.
Farrar JT, Messina J, Xie F, et al. A novel 12-week study, with three randomized double-blind placebo-controlled periods to evaluate fentanyl buccal tablets for the relief of breakthrough pain in opioid-tolerant patients with non-cancer-related chronic pain. Pain Med. 2010;11:1313–27.
Fine PG, Messina J, Xie F, et al. Long-term safety and tolerability of fentanyl buccal tablet for the treatment of breakthrough pain in opioid-tolerant patients with chronic pain: an 18-month study. J Pain Symptom Manag. 2010;40:747–60.
Webster LR, Messina J, Xie F, et al. Effect of fentanyl buccal tablet on pain-related anxiety: a 4-week open-label study among opioid-tolerant patients with chronic and breakthrough pain. J Opioid Manag. 2011;7:297–308.
Nalamachu S, Narayana A, Janka L. Long-term dosing, safety, and tolerability of fentanyl buccal tablet in the management of non-cancer-related breakthrough pain in opioid-tolerant patients. Curr Med Res Opin. 2011;27:751–60.
Portenoy R, Bruns D, Shoemaker B, et al. Breakthrough pain in community-dwelling patient with cancer pain and non-cancer pain. Part 2: impact on function, mood, and quality of life. J Opioid Manag. 2010;6:109–16.
Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21.
Trinidad JM, Herrera J, Rodríguez MJ, et al. Análisis de efectividad del citrato de fentanilo sublingual en pacientes con dolor irruptivo: estudio Sublime. Rev Soc Esp Dolor. 2011;18:207–18.
Überall MA, Müller-Schwefe GH. Sublingual fentanyl orally disintegrating tablet in daily practice: efficacy, safety and tolerability in patients with breakthrough cancer pain. Curr Med Res Opin. 2011;27:1385–94.
Gatti A, Reale C, Occhioni R, et al. Standard therapy with opioids in chronic pain management: Ortiber Study. Clin Drug Invest. 2009;29(suppl.1):17–23.
Gatti A, Reale C, Occhioni R, et al. Effects of opioid rotation in chronic pain patients: Ortibarn Study. Clin Drug Invest. 2010;30(Suppl 2):39–47.
Acknowledgments
Assistance with writing and preparation of this manuscript was provided by Maria José Sánchez, librarian at Hospital Universitari Sagrat Cor, Barcelona. No external funding was received to conduct the study. Data in this article were previously presented as poster at the XII Annual Meeting of the Spanish Society of Pain and XII Atlantic Islands Pain Forum 2011. The authors have no potential conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guitart, J., Vargas, I., De Sanctis, V. et al. Efficacy and Safety of Sublingual Fentanyl Orally Disintegrating Tablets in Patients with Breakthrough Pain: Multicentre Prospective Study. Clin Drug Investig 33, 675–683 (2013). https://doi.org/10.1007/s40261-013-0111-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0111-z