Abstract
Background
Chronic obstructive pulmonary disease (COPD) has a significant negative impact on quality of life and increases the risk of premature death. Umeclidinium is a long-acting muscarinic receptor antagonist in development for the treatment of COPD with the aim to broaden treatment options for clinicians and patients by providing improved symptom control.
Objective
To characterize the safety, tolerability, pharmacokinetics and pharmacodynamics of single and repeat inhaled doses of umeclidinium in healthy subjects.
Study Design
Two randomized, placebo-controlled, ascending-dose studies were conducted in healthy ipratropium bromide-responsive subjects. In the single-dose study, subjects (n = 20) received umeclidinium (10–350 μg), tiotropium bromide 18 μg and placebo in a crossover dosing schedule. In this study, lung function was assessed for 24 h by measuring specific airways conductance (sGaw) and forced expiratory volume in 1 s (FEV1). In the repeat-dose study, subjects (n = 36) received umeclidinium (250–1,000 μg) and placebo for 14 days in a parallel-group schedule.
Results
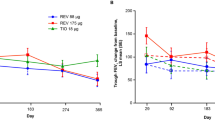
Adverse events (AEs) were reported in five subjects (single-dose study) and 23 subjects (repeat-dose study); none were serious. In both studies, no abnormalities in 12-lead electrocardiogram parameters, 24-h Holter monitoring or lead II monitoring were reported as AEs. Umeclidinium was rapidly absorbed following single-dose administration [time to reach the maximum plasma concentration (tmax) 5–15 min] and repeat-dose administration (tmax 5–7 min). Following repeat dosing, the geometric mean plasma elimination half-life was approximately 27 h and statistically significant accumulation was observed for the area under the plasma concentration–time curve, maximum plasma concentration and cumulative amount of unchanged drug excreted into the urine at 24 h (range 1.5- to 4.5-fold). Umeclidinium at doses of 100 μg and above, and tiotropium bromide demonstrated statistically significant bronchodilatory effects relative to placebo at 12 h post-dosing, which lasted up to 24 h for umeclidinium 350 μg and for tiotropium bromide. Relative to placebo, these increases in sGaw were 24–34 % at 12 h post-dose and 13 % at 24 h post-dose. Increases in FEV1 achieved statistical significance at 12 and 24 h for umeclidinium 100 μg and 350 μg compared with placebo.
Conclusion
Umeclidinium was well tolerated and demonstrated bronchodilatory effects in healthy subjects for up to 24 h. These bronchodilatory effects can be potentially clinically important in patients with airway obstruction such as COPD. The data obtained have informed dose selection for subsequent trials in subjects with COPD.



Similar content being viewed by others
References
Karner C, Cates CJ. Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(3):CD008532.
Russell R, Anzueto A, Weisman I. Optimizing management of chronic obstructive pulmonary disease in the upcoming decade. Int J Chron Obstruct Pulmon Dis. 2011;6:47–61.
Shavelle RM, Paculdo DR, Kush SJ, et al. Life expectancy and years of life lost in chronic obstructive pulmonary disease: findings from the NHANES III Follow-up Study. Int J Chron Obstruct Pulmon Dis. 2009;4:137–48.
From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011. http://www.goldcopd.org/. Accessed 17 Jan 2012.
Burge PS, Calverley PM, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–303.
Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–8.
Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–65.
Lainé DI, Luttmann MA, Foley JJ, et al. The pre-clinical pharmacology of the inhaled muscarinic antagonist GSK573719 predicts once-daily clinical dosing. Eur Respir J. 2011;38(Suppl. 55):3450.
World Medical Association. Declaration of Helsinki – ethical principles for medical research involving human subjects. Version October 1996. http://www.wma.net/en/30publications/10policies/b3/. Accessed 12 Dec 2011.
Algate C, et al. Umeclidinium (GSK573719) investigator’s brochure. Research Triangle Park: GlaxoSmithKline; 2012 (Data on file).
Singh D, Tal-Singer R, Faiferman I, et al. Plethysmography and impulse oscillometry assessment of tiotropium and ipratropium bromide; a randomised, double blind, placebo controlled, crossover study in healthy subjects. Brit J Clin Pharm. 2006;61:398–404.
Caillaud D, Le Merre C, Martinat Y, et al. A dose-ranging study of tiotropium delivered via Respimat Soft Mist Inhaler or HandiHaler in COPD patients. Int J Chron Obstruct Pulmon Dis. 2007;2:559–65.
Kesten S, Jara M, Wentworth C, et al. Pooled clinical trial analysis of tiotropium safety. Chest. 2006;130:1695–703.
Salpeter SR. Do inhaled anticholinergics increase or decrease the risk of major cardiovascular events? A synthesis of the available evidence. Drugs. 2009;69:2025–33.
Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:1439–50.
Agh T, Inotai A, Meszaros A. Factors associated with medication adherence in patients with chronic obstructive pulmonary disease. Respiration. 2011;82:328–34.
Acknowledgments
The authors would like to thank the subjects who participated in the studies; the staff at Parexel International GmbH (Berlin, Germany) for conducting the studies; and Amy Newlands and Alison Donald for their contributions to the study analyses. This work was presented, in part, at the European Respiratory Society (ERS) Annual Congress, Amsterdam, The Netherlands, 24–28 September 2011. Justin Cook, PhD, and Severina Moreira, PhD, of Niche Science and Technology Ltd (Richmond-upon-Thames, UK) provided writing and editorial support during the preparation of this manuscript; GlaxoSmithKline paid for these services. These studies were funded by GlaxoSmithKline.
Isabelle Pouliquen participated in the conception and design of both studies and in the analysis and interpretation of the data from one of the studies. Amanda Deans and Rashmi Mehta contributed to the analysis and interpretation of the data from one of the studies. Anthony Cahn and Kelly Hardes participated in the conception and design of the studies, and in the analysis and interpretation of the data. Ruth Tal-Singer, Andrew Preece and Glenn Crater contributed to the analysis and interpretation of the data. All authors have made critical revisions of draft versions of the manuscript and approved the final manuscript. All authors are GlaxoSmithKline employees.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cahn, A., Tal-Singer, R., Pouliquen, I.J. et al. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Single and Repeat Inhaled Doses of Umeclidinium in Healthy Subjects: Two Randomized Studies. Clin Drug Investig 33, 477–488 (2013). https://doi.org/10.1007/s40261-013-0088-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0088-7