Abstract
Background
Bioassays are used to identify the pharmacological activity of new or chemically unknown compounds, as well as their undesirable effect, including toxicity. Biological assays are also required to ensure the quality, safety, and efficacy of recombinant biologics to confirm its biosimilarity to its originator. In the present study, analytical similarity between the biosimilar and its innovator is established by in vitro bioassays.
Objective
The objective of this study was to show the comparative in vitro characterization of the recombinant insulin aspart from BioGenomics with its originator insulin aspart, using relevant biological assays.
Methods
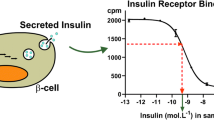
In vitro assays such as receptor binding, receptor autophosphorylation, glucose uptake, and mitogenic potential were analyzed for biological characterization of BioGenomics recombinant insulin aspart (BGL-ASP) manufactured by BioGenomics Limited and NovoRapid® as the reference medicinal product (RMP) manufactured by Novo Nordisk. Insulin receptor binding was studied by a state-of-the-art method, surface plasmon resonance (SPR) for biomolecular interactions. The receptor autophosphorylation assay measures the phosphorylated insulin receptor in cell lysates. The glucose uptake assay measures the uptake of glucose by 3T3-L1 cells in the presence of insulin. Lipogenesis was studied in treated 3T3-L1 cells by detecting the accumulation of lipid droplets in the cells. Mitogenic effect was studied by cell proliferation assay using MCF-7 cells. A rabbit bioidentity test was performed by measuring the sudden decrease in blood glucose in the presence of insulin.
Results
The binding studies showed that the affinity of BGL-ASP was highly comparable to NovoRapid®. Insulin receptor autophosphorylation, glucose uptake, and lipogenesis demonstrated high similarity to the RMP. The mitogenic assay for BGL-ASP did not show any proliferative effect and was comparable to the RMP. The in vivo bioidentity test showed that the BGL-ASP is highly similar to the innovator, NovoRapid®.
Conclusion
The biological characterization studies of BGL-ASP demonstrated high binding and functional similarity to NovoRapid®.





Similar content being viewed by others
References
Ghosh S, Bose S, Gowda S, Mukhopadhyay P. Biosimilar insulins—what a clinician needs to know? Indian J Endocrinol Metab. 2019;23(4):400–6. https://doi.org/10.4103/ijem.IJEM_180_19.
Dolinar RO, Edelman S, Heinemann L, Home P, Goyal S, Polonsky WH, Schellekens H. Impact of biosimilar insulins on clinical practice: meeting report. J Diabetes Sci Technol. 2014;8(1):179–85. https://doi.org/10.1177/1932296813518267.
Rotenstein LS, Ran N, Shivers JP, Yarchoan M, Close KL. Opportunities and challenges for biosimilars: what’s on the horizon in the global insulin market? Clin Diabetes. 2012;30(4):138–50. https://doi.org/10.2337/diaclin.30.4.138.
Kirchhoff CF, Wang XM, Conlon HD, Anderson S, Ryan AM, Bose A. Biosimilars: key regulatory considerations and similarity assessment tools. Biotechnol Bioeng. 2017;114(12):2696–705. https://doi.org/10.1002/bit.26438.
Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant human insulin and insulin analogues, EMEA/CHMP/BMWP/32775/2005_Rev. 1. https://www.ema.europa.eu/documents/scientific-guideline/guideline-non-clinical-clinical-development-similar-biological-medicinal-products-containing_en-0.pdf. Accessed 26 Feb 2015.
Reflection paper on statistical methodology for the comparative assessment of quality attributes in drug development, EMA/CHMP/138502/2017. https://www.ema.europa.eu/documents/scientific-guideline/reflection-paper-statistical-methodology-comparative-assessment-quality-attributes-drug-development_en.pdf. Accessed 26 July 2021.
Nupur N, Joshi S, Gulliarme D, Rathore AS. Analytical similarity assessment of biosimilars: global regulatory landscape, recent studies and major advancements in orthogonal platforms. Front Bioeng Biotechnol. 2022;10:832059. https://doi.org/10.3389/fbioe.2022.832059.
Tsong Yi, Dong X, Shen M. Development of statistical methods for analytical similarity assessment. J Biopharm Stat. 2015. https://doi.org/10.1080/10543406.2015.1092038.
Westermeier F, Sáez T, Arroyo P, Toledo F, Gutiérrez J, Sanhueza C, Pardo F, Leiva A, Sobrevia L. Insulin receptor isoforms: an integrated view focused on gestational diabetes mellitus. Diabetes Metab Res Rev. 2016;32(4):350–65. https://doi.org/10.1002/dmrr.2729.
Moruzzi N, Lazzeri-Barcelo F, Valladolid-Acebes I, Moede T, Paschen M, Leibiger B, Berggren PO, Leibiger IB. Tissue-specific expression of insulin receptor isoforms in obesity/type 2 diabetes mouse models. J Cell Mol Med. 2021;25(10):4800–13. https://doi.org/10.1111/jcmm.16452.
Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586–623. https://doi.org/10.1210/er.2008-0047.
Ward CW, Lawrence MC. Landmarks in insulin research. Front Endocrinol. 2011. https://doi.org/10.3389/fendo.2011.00076.
Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol. 2011. https://doi.org/10.1530/jme-11-0022.
Di Camillo B, Carlon A, Eduati F, Toffolo GM. A rule-based model of insulin signalling pathway. BMC Syst Biol. 2016. https://doi.org/10.1186/s12918-016-0281-4.
Hall C, Yu H, Choi E. Insulin receptor endocytosis in the pathophysiology of insulin resistance. Exp Mol Med. 2020. https://doi.org/10.1038/s12276-020-0456-3.
Belfiore A, Malaguarnera R, Vella V, Lawrence CM, Sciacca L, Frasca F, Morrione A, Vigneri R. Insulin receptor isoforms in physiology and disease: an updated view. Endocr Rev. 2017;38:379–431.
Sakoda H, Ogihara T, Anai M, Funaki M, Inukai K, Katagiri H, Fukushima Y, Onishi Y, Ono H, Fujishiro M, Kikuchi M, Oka Y, Asano T. Dexamethasone-induced insulin resistance in 3T3-L1 adipocytes is due to inhibition of glucose transport rather than insulin signal transduction. Diabetes. 2000;49:1700–8.
Subramanian K, Fee CJ, Fredericks R, Stubbs RS, Hayes MT. Insulin receptor-insulin interaction kinetics using multiplex surface plasmon resonance. J Mol Recognit. 2013;26:643–52.
Subramanian K. Kinetics of insulin–insulin receptor interaction using a surface plasmon resonance. 2014. http://hdl.handle.net/10092/9327. Accessed 05 Dec 2013.
Kleiman LB, Maiwald T, Conzelmann H, Lauffenburger DA, Sorger KP. Rapid phospho-turnover by receptor tyrosine kinases impacts downstream signaling and drug binding. Mol Cell. 2011;43:723–37.
Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Investig. 1995;95:2195–204.
Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278–88.
Murakami SM, Rosen MO. The role of insulin receptor autophosphorylation in signal transduction. J Biol Chem. 1991;266(33):22653–60.
Vishwanath D, Srinivasan H, Patil MS, Seetarama S, Agrawal SK, Dixit MN, Dhar K. Novel method to differentiate 3T3 L1 cells in vitro to produce highly sensitive adipocytes for a GLUT4 mediated glucose uptake using fluorescent glucose analog. J Cell Commun Signal. 2013;7:129–40.
Zhang LM, Hu JJ, Du G. Establishment of a cell-based assay to screen insulin-like hypoglycemic drugs. Drug Discov Ther. 2008;24:229–33.
Madsen L, Petersen RK, Kristiansen K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim Biophys Acta. 2005;1740:266–86.
Krycer JR, Quek LE, Francis D, Zadoorian A, Weiss FC, Cooke KC, Nelson ME, Diaz-Vegas A, Humphrey SJ, Scalzo R, Hirayama A, Ikeda S, Shoji F, Suzuki K, Huynh K, Giles C, Varney B, Nagarajan SR, Hoy AJ, Soga T, Meikle PJ, Cooney GJ, Fazakerley DJ, James DE. Insulin signaling requires glucose to promote lipid anabolism in adipocytes. J Biol Chem. 2020;295(38):13250–66.
Hui C, Zhang ZW, Guo Y, Wang Y, Liu Y, Luo N, Zhu Y. The proliferative role of insulin and the mechanism underlying this action in human breast cancer cell line MCF-7. J BUON. 2012;17:658–62.
Tennagels N, Werner U. The metabolic and mitogenic properties of basal insulin analogues. Arch Physiol Biochem. 2013;119(1):1–14.
Wei ML, Duan P, Wang ZM, Ding M, Tu P. High glucose and high insulin conditions promote MCF-7 cell proliferation and invasion by upregulating IRS1 and activating the Ras/Raf/ERK pathway. Mol Med Rep. 2017;16(5):6690–6.
Milazzo G, Giorgino F, Damante G, Sung C, Stampfer MR, Vigneri R, Goldfine ID, Belfiore A. Insulin receptor expression and function in human breast cancer cell lines. Cancer Res. 1992;52:3924–30.
USP-NF 〈121〉 INSULIN ASSAYS. https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/gc_121_insulin_assays_03012015.pdf. Accessed 01 May 2019.
Yie J, Dey M, Su J, Sergi J, Zhang Y, Le TH, Kashi S, Gurney K. Development of a robust functional cell-based assay for replacing the rabbit blood sugar bioidentity test of insulin glargine drug substance. J Pharm Biomed Anal. 2020;186: 113328. https://doi.org/10.1016/j.jpba.2020.113328.
Acknowledgements
We thank Dr. K. R. Srinivasan for the support and guidance throughout the process of submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was partially funded by USV Pvt. Ltd., India.
Conflicts of interest
All authors are employed by BioGenomics Ltd.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
AGM and RBD contributed towards the experimental setup, analysis, and interpretation of the data. DKT, NSG, and JL contributed in conception, design and development of the molecule. SMS and ARK contributed in development of the molecule, reviewed the article critically for important intellectual content, and gave their final approval of the version to be published.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, A.G., Deshmane, R.B., Thappa, D.K. et al. In Vitro Biological Characterization of Recombinant Insulin Aspart from Biogenomics and Originator Insulin Aspart. BioDrugs 37, 709–719 (2023). https://doi.org/10.1007/s40259-023-00607-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-023-00607-4