Abstract
Acute respiratory distress syndrome (ARDS) is a severe respiratory disease associated with high morbidity and mortality in the clinic. In the face of limited treatment options for ARDS, extracellular vesicles derived from mesenchymal stem cells (MSC-EVs) have recently shown promise. They regulate levels of growth factors, cytokines, and other internal therapeutic molecules. The possible therapeutic mechanisms of MSC-EVs include anti-inflammatory, cell injury repair, alveolar fluid clearance, and microbe clearance. The potent therapeutic ability and biocompatibility of MSC-EVs have enabled them as an alternative option to ameliorate ARDS. In this review, recent advances, therapeutic mechanisms, advantages and limitations, as well as improvements of using MSC-EVs to treat ARDS are summarized. This review is expected to provide a brief view of the potential applications of MSC-EVs as novel biodrugs to treat ARDS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Extracellular vesicles derived from mesenchymal stem cells (MSC-EVs) can attenuate acute respiratory distress syndrome (ARDS) due to their anti-inflammatory and cell repair properties. |
Biotechnological processing and appropriate administration routes can further improve the therapeutic effect of MSC-EVs and overcome their limitations. |
1 Introduction
Acute respiratory distress syndrome (ARDS) is an acute and severe condition characterized by excessive alveolocapillary permeability and pulmonary edema [1]. ARDS was first reported in 1967 based on symptoms of hypoxia, pulmonary edema, and alveolocapillary membrane injury [2]. Inflammatory and immune dysregulation in ARDS results in the release of various cytokines and chemokines [3]. Sepsis, multiple traumas, pneumonia, and inhalation of toxic gases can cause ARDS, which affects about 3 million people each year, and 24% patients receive mechanical ventilation [4]. The associated mortality rate is still up to 40% [5, 6]. With the outbreak of the coronavirus disease 2019 (COVID-19) pandemic in 2019, pulmonary inflammatory diseases have garnered much attention. COVID-19 and other infections, such as H1N1, may both accompany ARDS as acute respiratory symptoms are the initial characteristics of these conditions [7]. Thus, differentiating ARDS induced by COVID-19 from ARDS induced by other infections is important for clinicians and epidemiologists. A retrospective study showed that patients with COVID-19 were more prone to nonproductive cough and constitutional symptoms [8]. Fever, dry cough, and obvious fatigue are more common in COVID-19 patients [9]. Patients with H1N1 exhibited productive cough and rhinorrhea [10]. Hence, ARDS induced by COVID-19 may more likely present as obvious systemic symptoms with fewer productive coughs and slow onset. Ground-glass opacity is also more common in radiologic results of patients with COVID-19 than patients with H1N1 [11]. In addition to diffuse alveolar damage, cellular fibromyxoid exudates on pulmonary pathologic findings are common in COVID-19 [12]. Ackermann et al. [13] have reported more intussusceptive angiogenesis in samples of COVID-19 than other influenzas. However, H1N1 involved extensive hemorrhage and necrotizing bronchiolitis [14]. More importantly, the severity of respiratory failure is not the same. Patients with COVID-19 had higher levels of arterial oxygen tension to inspiratory oxygen fraction ratio (PaO2/FiO2 ratio)[2]. More severe respiratory failure may translate to higher mortality. Therefore, early specific respiratory support is beneficial for patients. Patients with ARDS usually have a poor quality of life and reduced exercise ability. Moreover, they have an increased risk of readmission, burdening the public health system [15,16,17].
ARDS is commonly accompanied by an acute pro-inflammatory response, making it crucial to inhibit the pro-inflammatory response and regulation of immune cells [18]. Continuous inflammation will injure the alveolar epithelial–endothelial barrier and cause alveolar fluid accumulation, which will eventually impair gas exchange and result in hypoxemia [19]. Effective therapeutics to treat ARDS remain to be identified [20]. Although mechanical ventilation, neuromuscular blockade, and extracorporeal membrane oxygenation have been applied clinically, the outcomes are usually unsatisfactory and even lead to disease progression. For example, mechanical ventilation increases the risk of ventilation-induced lung injury due to epithelial strain and stress [21, 22]. Treatment options for ARDS are limited. Several strategies, such as use of anti-oxidants, surfactants, and nitric oxide, have not been satisfactory. Thus, more specific and compelling methods are required for ARDS therapy.
Recently, mesenchymal stem cells (MSCs) have demonstrated promising potential as living biodrugs to counter inflammation and dysregulated immune activation [23]. This immune regulation ability and the differentiation capability of MSCs make them a promising treatment for ARDS restoration. However, several inherent limitations, such as uncontrollable cell differentiation, proliferation, and low engraftment, restrict their clinical application potential to treat ARDS [24]. Extracellular vesicles (EVs) produced by MSCs (MSC-EVs) are reported to inherit some biofunctions of MSCs, and have reduced safety risks and improved pharmacokinetics in circulation compared to MSCs [25]. Therefore, MSC-EVs, as a substitute of MSCs, can be used for ARDS treatment. MSC-EVs from bone marrow-derived MSCs alleviate lung injury, through their anti-inflammatory and immunomodulatory effects [26]. MSC-EVs could reduce the severity of ARDS by inhibiting microbial activity [27]. They alleviate damage to multiple cell types associated with lung injury, including immunocytes, microvascular endothelial cells, and alveolar epithelial cells. Hence, they can be used to treat ARDS through their anti-inflammatory, cell injury repair, alveolar fluid clearance, and anti-microbial activities [28].
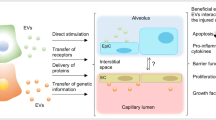
In addition to their inherent therapeutic potential, MSC-EVs can also act as biocarriers for delivering therapeutic drugs for synergistic effects. MSC-EVs carry various “cargos,” including nucleic acids, lipids, and proteins. These cargos will be delivered to target cells and exert bioactivities [29]. Such a strategy may allow for precise and cell- or tissue-selective delivery of drugs into the patient’s bloodstream via MSC-EVs [29]. In the present review, the biological effects, therapeutic mechanisms, and potential applications of MSC-EVs for ARDS treatment are summarized. In addition, challenges and improvements in using MSC-EVs as both biodrugs and biocarriers for ARDS treatment are addressed. This review is expected to provide new ideas to overcome current obstacles in ARDS treatment (Fig. 1).
A schematic illustration of extracellular vesicles used for acute respiratory distress syndrome treatment. The therapeutic effects of mesenchymal stem cells on damaged lung tissue are mainly achieved through secreted extracellular vesicles. Four main mechanisms are involved, including anti-inflammatory, cell injury repair, alveolar fluid clearance, and microbe clearance
2 ARDS Treatment Using MSCs and the Therapeutic Mechanisms
2.1 MSC-Based Therapy
MSCs are undifferentiated cells, first isolated from bone marrow by Friedenstein et al. in the 1970s [30]. They are abundantly present early in life and continuously produce differentiated cells during development, constituting tissues and organs [31]. They have a relatively short lifespan, which shortens retention time and partly decreases the risks of immunogenicity in vivo. These properties make MSCs suitable for ARDS treatment [32]. MSCs have been used to treat lung injury in the preclinical settings [33,34,35,36,37]. Of the 42 registered clinical trials on ARDS treatment, seven have been completed, according to the US National Institutes of Health (https://clinicaltrials.gov, NCT01775774, NCT02804945, NCT04625738, NCT04355728, NCT04493242, NCT04333368, NCT04400032). These trials reveal the promising potential of MSC-based therapy for ARDS treatment. However, there is no consensus on the administration dose, route, and source of MSCs in the clinical settings [38]. A randomized phase 2a trial demonstrated that one dose of allogeneic MSCs is safe and effective for ARDS patients [39]. Compared with the placebo group, the MSC-treated group showed improved therapeutic effect regarding the numerical value of Acute Physiology and Chronic Health Evaluation III, minute ventilation, and positive end-expiratory pressure [39]. A double-blind, phase 1/2a, randomized, controlled trial with safety in 6 h, patient survival at 31 days, and time to recovery as endpoints showed no difference between the treatment group and control group in adverse events. Administration of MSCs significantly improved patient survival and decreased inflammatory cytokines on day 6 [40]. Thus, MSC therapy has made some progress in clinical trials, showing safety and potential efficacy. The therapeutic effects are associated with the ability of MSCs to detect an injury microenvironment and promote tissue regeneration [41]. MSCs possess several advantages in ARDS treatment. Firstly, due to their immunomodulatory ability, MSCs inhibit the excessive inflammatory responses in ARDS, protecting the pulmonary tissue from injury [42]. Secondly, MSCs promote microbe clearance, thereby accelerating recovery from bacterial infection, which is the leading cause of ARDS [43]. Finally, MSCs may induce alveolar stem or progenitor cells to differentiate into alveolar endothelial cells [44]. MSCs can also directly differentiate into alveolar endothelial cells, which may further benefit the reparation of the injured alveolar cells. [45,46,47]. However, this mechanism of pulmonary injury repair needs to be studied further.
Nevertheless, the safety and efficacy of MSC-based therapies remain to be established for ARDS. MSCs can target other tissues and alter the microenvironment; MSC-secreted hepatocyte growth factor may have a risk of inducing liver cancer [48]. In addition, MSCs can also differentiate into cancer-associated fibroblasts and promote tumor growth and invasion [49]. Although MSCs have not been found to form tumors in clinical trials, the risk requires further studies before MSCs are applied to patients [50]. Secondly, MSCs have been demonstrated to present antigens when stimulated by some factors and may cause immune elimination or other responses [51]. For instance, the expression of major histocompatibility complex (MHC) II can be induced by a low concentration of interferon (IFN)-λ and by MSCs when stimulated by a low concentration of transforming growth factor (TGF)-β or high MSC culture density [52]. Thirdly, several studies showed low engraftment rates after administration of MSCs in vivo, mainly because of inflammation, hypoxia, and oxidative stress, resulting in poor regenerative effects of MSCs on injured tissue [53,54,55,56].
2.2 Roles of MSC-EVs
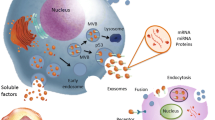
The therapeutic and regenerative effects of MSCs are closely associated with growth factors, cytokines, chemokines, cell adhesion molecules, hormones, lipid mediators, interleukins (ILs), and EVs [57,58,59,60]. EVs are small membrane-bound or anuclear microparticles released by eukaryotic cells [61]. As a highly heterogeneous population, EVs can induce a complex response. They are generally divided into two categories, ectosomes and exosomes. Ectosomes are formed through the direct outward budding of plasma membrane in the size range of 50–1000 nm. Exosomes originate from endosomes and are 100 nm on average [62]. The more detailed classification of EVs may include exosomes, microvesicles, and apoptotic bodies (Table 1) [63]. In the present review, EVs refer more to exosomes and microvesicles generated by MSCs.
EVs have demonstrated treatment potential and play crucial roles in the intercellular communication between MSCs and injured cells, which may confer them the ability to transfer bioactive contents between donor cells and target cells. EVs interact with the target cell by receptor-mediated binding [58]. The bonded EVs can release their internal contents by cell membrane fusing or by direct endocytosis [64]. In addition, some ligands on EVs can bind to specific receptors on the target cells and active target cells through the downstream signal pathway [65].
Additionally, EVs can carry diverse cargos, including lipids, various RNAs, multiple species of proteins, and even organelles such as mitochondria [25, 66, 67]. These substances can alter the epigenetic environment and expression of proteins within target cells. The proteins delivered by EVs can target specific intracellular mechanisms, and the RNAs transferred by EVs may induce the translation of mRNAs or modulate other translation processes [68]. EVs enriched with microRNA (miR)-210 could target the angiogenesis gene Efna3, promoting human umbilical vein endothelial cell (HUVEC) proliferation and capillary-like structure formation to accelerate vascular regeneration [69]. EVs derived from atorvastatin-pretreated MSCs could upregulate miR-221-3p expression by activating the protein kinase B and endothelial nitric oxide synthase (AKT/eNOS) pathway and repair the injured pulmonary tissue [70].
ARDS also causes various organ dysfunction syndromes, grouped as mitochondrial dysfunction [71]. EVs can convey complex information to recipient cells through mitochondrial transfer, improving the bacterial clearance capacity of alveolar macrophages [72]. Thus, EVs carrying mitochondria may improve the mitochondrial function of recipient cells and ARDS treatment [73].
For ARDS treatment, a lack of sufficient relevant studies makes it challenging to conclude whether EVs from different cell sources exhibit functional differences. However, it is possible that MSCs from different sources may differ in their mechanism of tissue repair. Periera-Simon et al. [35] examined the anti-fibrotic effects of MSCs derived from adipose tissue, chorionic membrane, chorionic villi, and Wharton’s jelly. Except for chorionic membrane-derived MSCs, all MSCs decreased the hydroxyproline levels and tumor necrosis factor (TNF)-α mRNA expression levels after 10 days of treatment. Only MSCs derived from adipose tissue and Wharton’s jelly inhibited AKT and matrix metalloproteinase (MMP)-2 activation. Only adipose-derived MSCs rectified miR dysregulation. Antunes et al. [74] revealed that MSCs isolated from different mouse tissues exhibited different therapeutic effects. Bone marrow- and adipose-derived MSCs decreased the abundance of M1 macrophages and vascular endothelial growth factor levels. However, only bone marrow-derived MSCs increased the abundance of M2 macrophages. In summary, MSCs from different sources act via different pathways in pulmonary diseases. These studies show that the source of MSCs has impacts on the therapeutic mechanism and outcomes. Thus, EVs generated from different MSCs may have similar results, but still need more studies to support them. The biological effects of EVs from different sources of MSCs in ARDS treatment are summarized in Table 2.
Additionally, administration routes determine the biodistribution of EVs, which is essential for medical applications [75]. The intravenous route is the most common route of EV administration [76]. However, most EVs are distributed in the liver and are cleared by macrophages [77, 78]. Intraperitoneal and subcutaneous injection of EVs is also widely reported, with similar biodistribution effects. The accumulation of EVs in the spleen and gastrointestinal tract is higher than in the liver for these administration routes [79]. Some EVs are absorbed into the circulation after oral administration. EVs can also be given through oral administration for accumulation in gut [80] and colon [81]. Intranasal administration is a unique route for brain-targeted delivery, and EVs can rapidly accumulate in the brain [82, 83]. For ARDS treatment, the administration route with preferential pulmonary accumulation is ideal. Nebulization increases the accumulation of adipose-derived MSC-EVs in the lung of a mouse model of acute lung injury [84]. Zhang et al. [85] have demonstrated that EVs are specifically taken up by pulmonary macrophages after intratracheal administration. To conclude, intratracheal administration and nebulization are promising EV administration routes; they lead to accumulation in the lung and may improve therapeutic effects of EVs. However, more systematic research is required for further application of these potential administration routes.
EVs are more stable and have lower immunogenicity than the cells, addressing the shortcomings of MSC therapy for ARDS treatment [86]. These advantages in addition to the vital roles played by MSC-EVs in ARDS treatment provide a novel strategy of directly using MSC-EVs instead of MSCs to treat ARDS [87]. More mechanisms and therapeutic outcomes of using MSC-EVs for ARDS treatment will be introduced in the following.
3 Therapeutic Effects and Mechanisms of MSC-EVs in ARDS Treatment
The therapeutic mechanisms of MSC-EVs in ARDS are similar to MSC-based therapy, i.e., they exhibit anti-inflammatory, cell injury repair, alveolar fluid clearance, and microbe clearance (Fig. 2).
3.1 Immunomodulatory and Anti-inflammatory Effects
The exacerbated inflammatory response is the major pathologic event during ARDS progression. Therefore, inhibiting inflammation is critical in ARDS treatment. MSC-EVs reduce inflammation in sepsis [88, 89], periodontitis [90], renal tubular injury [91], and neuronal diseases [92]. MSC-EVs have been used for ARDS treatment because of their potent anti-inflammatory properties.
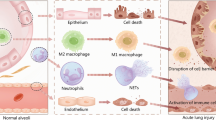
The anti-inflammatory effects of MSC-EVs are mainly associated with their ability to modulate levels of immune cells. For example, MSC-EVs target pro-inflammatory pathways and chemotaxis inhibiting the expression of immunoglobulin-related genes and decreasing pulmonary B cells in mice [93]. This immune modulation by MSC-EVs is related to the recruitment of anti-inflammatory neutrophils to the lung tissue. Di Trapani et al. [94] demonstrated that MSC-EVs can regulate the activation of T cells in lung tissues. The uptake of EVs by T cells would inhibit T cell activity. Chen et al. [95] observed that MSC-EVs induced the differentiation of T helper (Th) cells into Th type 2 cells. EVs extracted from healthy human bone marrow can inhibit the release of pro-inflammatory factors, including IL-1β and TNFα. EVs can also increase the secretion of anti-inflammatory factor TGF-β.
Notably, MSC-EVs can promote the transformation of the inflammatory M1 macrophage to the anti-inflammatory M2 phenotype. This polarization accelerates the anti-inflammatory cytokine release, including TGF-β, prostaglandin E2 (PGE2), and IL-10. Deng et al. [26] reported that MSC-EVs inhibit M1 rather than M2 polarization in ARDS development induced by sepsis. MSC-EVs also inhibited hypoxia inducible factor 1α (HIF-1α) secretion and downregulated several essential proteins of glycolysis expression, such as hexokinase 2 (HK2), pyruvate kinase isoform M2 (PKM2), and other proteins. The inhibitory effects significantly influenced the production of adenosine triphosphate (ATP) and lactic acid and regulated macrophage polarization. The acute inflammatory response was attenuated by inhibiting glycolysis. The in vivo experiment also revealed the ameliorative effect of the lipopolysaccharide (LPS)-induced ARDS. After treatment with MSC-EVs, the survival rate of mice was improved significantly, and the PaO2/FiO2 ratio also showed a satisfactory improvement. In addition to macrophages, MSC-EVs also regulate T cells. Lo Sicco et al. [96] reported that MSC-EVs can be efficiently internalized by macrophages, which promotes the macrophage phenotypes switching. Effector T cells secrete IL-17, which protects against microbial infections and the development of inflammatory disease. EVs increase this type of cytokine release by adjusting the ratio between the effector T cells and regulatory T cells.
Interestingly, Morrison et al. [18] showed that MSCs promote the phagocytic macrophage phenotype through EV-mediated mitochondrial transfer, leading to anti-inflammatory effects. MSCs stimulated by bronchoalveolar lavage fluid could release cluster of differentiation-44 (CD44)-expressing EVs, enhancing the phagocytic ability of M2 macrophages. LPS could enhance the phagocytic ability macrophages similar to bronchoalveolar lavage fluid, mainly by enhancing the oxidative phosphorylation in macrophages [18]. The treated macrophages ameliorate lung injury in vivo.
3.2 Repair of Epithelial and Endothelial Cell Injury
In addition to their anti-inflammatory capability, MSC-EVs repair injured microvascular endothelial and epithelial cells, which are the major cause of mortality induced by ARDS [97]. Hu et al. [98] observed that MSC-EVs protected lung microvascular endothelial cells from inflammatory injury. EVs transferred angiopoietin-1 (Ang-1) mRNA to recover normal protein permeability and avoid the formation of actin stress fibers. After transfer to the recipient cells, the mRNA induced expression of soluble factors to assist the restoration of microvascular endothelial permeability. Yu et al. [70] have demonstrated that EVs isolated from atorvastatin-pretreated MSCs have a pro-angiogenic ability. These EVs could activate the AKT/eNOS pathway and upregulate miR-221-3p expression, and the high expression of miR-221-3p accelerated the migration, proliferation, and formation of endothelial cells. Additionally, MSC-EVs also improved the angiogenesis of pulmonary endothelial cells. Wang et al. [69] revealed that the angiogenic effect of MSC-EVs was probably associated with enrichment of miR-210 by EVs. The angiogenesis gene Efna3, which can promote capillary-like tube formation and proliferation of HUVECs, is the target gene of miR-210. The formation and proliferation effects were considered key to the regeneration of pulmonary vascular cells.
In addition, MSC-EVs attenuate mitochondrial dysfunction to restore pulmonary microvascular endothelial cell injury. Dutra Silva et al. [99] reported that mitochondrial transfer relieves ARDS and promotes the repair of the alveolar epithelial-capillary barrier. MSC-EVs contain mitochondria, which are easily intercalated within the endothelial barrier. The mitochondrial transfer can alleviate mitochondrial dysfunction and barrier integrity reparation, as well as restore the level of mitochondrial biogenesis and respiration to normal. EVs without mitochondria are not effective. Thus, the mitochondrial transfer is essential for pulmonary injured endothelial cell recovery. More importantly, the author mentioned plasma in vivo could influence MSC-EVs’ therapeutic effects. Therefore, the application of MSC-EVs merited further research.
It is essential to restore alveolar epithelial cell functions for repairing lung tissue injury. Zhou et al. [100] observed that MSC-EVs could ameliorate cell damage and apoptosis of the rat renal proximal epithelial cell line NRK-52E. Cisplatin can cause apoptosis, necrosis, and oxidative stress in epithelial cells by activating p38 mitogen-activated protein kinase (p38MAPK) and caspase 3 upregulation. However, MSC-EVs could attenuate oxidative stress and apoptosis by attenuating the effect of cisplatin on the abnormal activation of p38MAPK and caspase 3 expression and by activating the extracellular signal-regulated kinase 1/2 pathway. The studies enhanced the antiapoptosis and antioxidation activity of MSC-EVs. This study verified the anti-apoptosis and antioxidant activity of MSC-EVs, making them useful for alveolar epithelial tissue regeneration. Similarly, Monsel et al. [101] have demonstrated that MSC-EVs could deliver mitochondria to injured alveolar epithelium and increase the survival and intracellular ATP generation of alveolar epithelium. Moreover, intravenous injection of MSC-EVs can improve the keratinocyte growth factor (KGF)-mediated cell survival effect and decrease TNFα secretion in the setting of LPS-primed injury of alveolar epithelial type 2 cells. The therapeutic effects on other organs have also been demonstrated [99, 102, 103].
Thus, many studies have demonstrated that increased levels of growth factors, extracellular matrix proteins, and anti-inflammatory proteins are involved in cell and tissue regeneration [104]. MSC-EVs transfer mitochondria, mRNA, and miR to regulate intercellular signaling pathways. MSC-EVs can enhance the proliferation and migration of epithelial and endothelial cells, thus ameliorating ARDS.
3.3 Promotion of Alveolar Fluid Clearance
Patients with ARDS usually have varying degrees of pulmonary edema. Hence, promoting alveolar fluid clearance is also critical to ameliorate ARDS [105]. EVs secreted from MSCs promoted the alveolar fluid clearance, thereby improving the recovery of lung injury after ARDS. Gennai et al. [106] demonstrated that MSC-EVs enhanced pulmonary edema fluid clearance in a dose-dependent manner, which attenuated lung weight gain after ventilation and perfusion. They also proved that CD44 improves airway and hemodynamic parameters. Administration of anti-CD44 antibody attenuated the internalization and therapeutic effects of EVs. The protection of injured lung cells also indicated the use of MSC-EVs in rehabilitating human donor lung. However, the mechanism of alveolar fluid clearance needs further research. Loy et al. [107] demonstrated that MSC-EVs restored the expression of epithelial ion transporters and alveolar fluid clearance during influenza virus infection. Alveolar fluid clearance was attenuated by inhibition of transporter protein expression and damage to tight junctions.
Umbilical cord-derived MSC-EVs expressed high levels of angiogenesis and pulmonary tissue remodeling factors, essential for alveolar fluid clearance [108]. MSC-EVs reduced lung protein permeability and increased alveolar fluid clearance by promoting the release of fibroblast growth factor (FGF)-7. Monocyte-derived macrophages in bronchoalveolar lavage fluid promote cytokine production, creating an inflammatory microenvironment in the alveolar fluid. Intravenous administration of MSC-EVs decreased the number of neutrophils and other inflammatory cells. MSC-EVs significantly decreased bacterial colony-forming units and reduced the inflammation of injured lung tissue induced by Escherichia coli (E. coli). Polyinosinic-polycytidylic acid could improve the therapeutic effects of MSC-EVs on alveolar fluid clearance, but had a minor impact on lung oxygenation, compliance, and tracheal pressure. Promoting alveolar fluid clearance can accelerate anti-inflammation to promote recovery from ARDS [109, 110].
To conclude, the promotion of alveolar fluid clearance promotes recovery of injured lung tissue in severe ARDS. MSC-EVs can transfer intercellular components, such as FGF and KGF, to restore lung protein permeability. These components can upregulate alveolar fluid transport and restore lung protein permeability. Thus, alveolar fluid clearance is improved and benefits ARDS treatment.
3.4 Antimicrobial Properties
A considerable number of ARDS cases are induced by bacterial pneumonia and influenza virus [1]. Acute microbial infections can cause severe inflammation and destruction of pulmonary tissues, which can eventually develop into ARDS [111]. Therefore, the clearance of microbes is critical for the treatment of bacteria- or virus-induced ARDS.
MSC-EVs can transfer immunoregulatory substances, including proteins, RNAs, and lipids, to recipient cells and have a similar regulatory effect to MSCs [112]. MSCs can improve innate immune cell function. LL-37, β-defensin-2 (BD2), and other antimicrobial factors are vital for bacterial clearance [113,114,115]. LL-37 has antimicrobial properties as an essential part of the innate immune system. LL-37 is effective against various pathogens, including viruses, bacteria, and fungi [116, 117]. BD2 is a microbicidal paracrine mediator secreted by MSCs [115]. These active antibacterial substances can be loaded into EVs to achieve antibacterial effects. MSC-EV treatment increased the phagocytosis of alveolar macrophages, enhancing microbial clearance [108]. Such antibacterial effects of MSC-EVs have also been observed in an E. coli-induced infection model. Hao et al. [27] showed that MSC-EVs enriched with miR-145 increase the production of leukotriene B4 and decrease the activity of multidrug resistance-associated protein 1 (MRP1) in monocytes. Enhanced phagocytosis and release of antimicrobial agents by monocytes result in antimicrobial effects. Monsel et al. [101] found that MSC-EVs can deliver antimicrobial substances to decrease the number of inflammatory cells and cytokines. MSC-EVs delivered KGF and enhanced the phagocytosis of monocytes, enhancing the antimicrobial effects of the host immune system. CD44 receptors play a crucial role in the cellular uptake of MSC-EVs by monocytes. CD44-neutralizing antibodies could abrogate the uptake of MSC-EVs and decrease mouse survival, affecting ARDS treatment [101].
Viral inhibition also contributes to the antimicrobial properties of MSC-EVs [18]. MSC-EVs upregulated bacterial phagocytosis by macrophages via mitochondrial transfer to monocytes and macrophages. This process might be associated with the increase of oxidative phosphorylation in human monocyte-derived macrophages, due to the enhanced phagocytic capacity [18]. In the case of viral infection-induced ARDS, the underlying mechanism of MSC-EVs attenuates viral replication and suppresses the cytokine storm in viral infection-induced ARDS [27]. The miRs derived from MSC-EVs could interact with the viral genome and influence viral protein expression to enhance the antiviral effects [118].
In summary, MSC-EVs can treat ARDS due to their ability to modulate the inflammatory response, induce the regeneration of injured alveolar epithelial endothelial cells, accelerate alveolar fluid clearance, and promote microbial clearance (Table 2). Nevertheless, the current application of natural MSC-EVs is still restricted by several innate limitations of MSC-EVs, such as the limited range of natural MSC-EVs’ internal components.
4 Methods to Improve the Therapeutic Efficacy of EVs in ARDS
One of the main limitations of natural MSC-EVs is the unsatisfying drug-loading capacity, which restricts their therapeutic efficiency [119]. Therefore, improving the loading capacity of MSC-EVs is a potential strategy to enhance the therapeutic effects of MSC-EVs. Several methods have been developed to enrich the natural and exogenous contents of MSC-EVs.
MSC-EVs are secreted by MSCs. Changing the surface and intracellular contents of cells could influence MSC-EVs efficacy [99]. Toll-like receptor 3 agonist preconditioning promoted anti-microbial activity and polarized monocytes toward the M2 anti-inflammatory phenotype [101]. IFN-γ-primed MSCs attenuated ARDS more effectively than naïve MSCs by promoting macrophage phagocytosis and clearance of microbes [120]. Furthermore, the immortalization of MSCs assisted their proliferation, prevented senility, and retained mesenchymal phenotype and multipotency for more passages [121, 122]. This strategy may allow MSCs to produce MSC-EVs with stable quality.
Loading of exogenous drugs can also improve the therapeutic potential of MSC-EVs. There were three major strategies to load exogenous drugs: pre-production, per-production, and post-production [123]. Pre-production loading methods allowed cargos, such as nanoparticles, nucleic acids, and molecular drugs, to be shipped into MSCs. Then the secreted EVs containing these therapeutic drugs will deliver them to the target cells. Pretreating MSCs with iron oxide nanoparticles (IONPs) could generate IONP-loaded EVs [124]. These MSC-EVs containing IONPs acted as a magnetism-guided navigation vehicle and resulted in the production of more growth factors through the c-Jun N-terminal kinase signaling cascade. In addition, the hydrophilic core and lipophilic membrane allowed EVs to encapsulate a huge amount of drug molecules with different properties. Silva et al. [125] also reported a hybrid vector for drug delivery via EVs. In addition to IONPs, EVs were loaded with doxorubicin (DOX), tissue-plasminogen activator, and photosensitizers. This drug delivery system has a potential application in ARDS treatment.
Genetic modification is another strategy to augment the therapeutic potential of MSC-EVs. PGE2 is an inflammatory cytokine that activates the E-prostanoid 2 (EP2) receptor and promotes the migration of MSCs [126]. Transducing MSC-based EP2 genes facilitated the mobilization of MSCs and improved the recovery of pulmonary inflammation and permeability [127]. As a cytoprotective enzyme, heme oxygenase-1 (HO-1) could attenuate pulmonary cell injury through its anti-inflammatory and antiapoptotic effects. Chen et al. [128] reported that HO-1-modified MSCs protect against ARDS progression. HO-1-modified MSCs were established by lentiviral transduction. These modified MSCs secreted MSC-EVs, significantly improved the survival of rats, and decreased neutrophil counts and total protein concentration in the bronchoalveolar lavage fluid. Hence, HO-1-modified MSC-EVs showed additional recovery and antiapoptotic properties compared with naïve MSC-EVs. Additionally, He et al. [129] focused on angiotensin-converting enzyme 2 (ACE2), which degrades angiotensin II to angiotensin (1-7) and protects against pulmonary tissue damage. Lentiviral vector transduction of the ACE2 gene into MSCs and administration of these MSCs into ACE2-knockout lung injury model mice resulted in histologic improvement in lung tissue and superior anti-inflammatory effects 72 h later. Although some of these studies focused on diseases other than ARDS, the therapeutic effects of MSC-EVs remain verified. The paracrine effects uphold the therapeutic potential of MSC-EVs.
The per-production strategy involves loading of drugs during EV production, with the destruction and reformation of the EV membrane structure. Toledano Furman et al. [130] reported a method to load TNF-related apoptosis-inducing ligand into MSC-EVs. They emptied the cytosolic content of MSCs by hypotonic shocking, sonicating the membrane, and extruded them through 0.4-μm polycarbonate membrane filters to load the cargo. Additionally, DOX and carboplatin were also loaded into EVs in a similar manner [131]. However, the process of destruction and reformation of EVs’ membrane structure may destroy the EV membrane and cause loss of internal bioactive substances, impairing the targeting and delivery properties of MSC-EVs.
With the post-production strategy, the most common approach is incubation, which could load lipophilic or hydrophilic compounds into EVs. The membrane structure and inner core of EVs allow loading of different hydrophilic and lipophilic drugs through incubation [132]. Lipophilic drugs are easier to fuse with the membrane and pack into EVs. Zhuang et al. [82] encapsulated lipophilic curcumin into EVs as a drug delivery system by simple incubation. Gong et al. [132] loaded DOX into EVs by incubation with 200 μg/mL of DOX and encapsulated 160 ng DOX per microgram of EVs, an acceptable encapsulation efficiency for lipophilic drugs. However, this might result in the fusion of excessive lipophilic substances and affect the properties of the EV membrane. In addition, exceptionally high-molecular-weight, hydrophilic, and nucleic drugs are difficult to load due to their inability to cross the EV membrane. Sonication and electroporation are two well-developed methods that could improve the encapsulation efficiency and loading capacity. Kaneti et al. [133] used high-energy sonication to introduce payloads into MSC-EVs. This strategy significantly increased the phospholipid yield and the lipid-to-protein ratio. Pomatto et al. [134] employed electroporation of EVs to load miR. By adjusting voltage and pulse, different payloads could be loaded into EVs and achieve better loading efficiency with less damage to the EVs.
Preconditioning stimulation is another major factor determining both the quantity and composition of EVs. Hypoxia-preconditioning could upregulate miR-612 in MSC-EVs, which stimulated angiogenesis by targeting the 3′-untranslated region of TP53 [135]. The enhanced angiogenesis effect may represent hypoxia-preconditioned MSC-EVs as potential drugs for regeneration. Duan et al. [136] isolated synovial MSCs to explore the therapeutic effects of LPS-stimulated MSC-EVs on chondrocytes in osteoarthritis. LPS-stimulated MSC-EVs significantly promoted the proliferation and migration of chondrocytes and decreased the osteoarthritis severity in a mouse model. In addition to the composition of MSC-EVs, the production also can be improved by magnetic stimulation. Wu et al. [137] stimulated MSCs by static magnetic fields to obtain MSC-EVs loaded with magnetic nanoparticles. The production of EVs increased by about 30% after magnetic nanoparticles and static magnetic fields stimulation. Enhanced miR-1260 expression also inhibits HDAC7 and COL4A2 expression to improve angiogenesis. Yang et al. [138] reported a cellular-nanoporation strategy to improve EV production, even from cells with low secreting levels of EVs. Electrical stimulation improved the production of EVs by 50-fold. Therefore, external stimulation can be a potential strategy to enhance the therapeutic abilities of EVs.
Taken together, several strategies have been reported to augment the therapeutic potential of MSC-EVs (Fig. 3). However, the application of these modified EVs to treat ARDS currently lacks robust evidence. The success of modified EVs in the treatment of other diseases may inspire the potential use of modified EVs for ARDS treatment.
5 Future Perspective
As a potential method of drug delivery, MSC-EVs have similar therapeutic effects to MSCs but without the potential risks of MSCs. MSC-EVs transfer various substances to recipient cells while possessing pro-regenerative and targeting properties [139]. Compared with MSC-based drug delivery systems, MSC-EVs have some unique advantages. Firstly, MSC-EVs are more controllable biodrugs than MSCs, with fewer safety concerns and more stability. Secondly, the storage of MSC-EVs is easier than that of MSCs, facilitating their practical clinical use. MSC-EVs can be stored in a refrigerator at −80 ℃ for clinical application and remain biologically active. This may avoid issues related to storage facilities in hospitals lacking facilities for storing a large number of cells. Finally, the nanosized MSC-EVs have less risk of elevating pulmonary arterial pressure or causing pulmonary embolism. Thus, multiple doses and high-dose administration may be considered safe.
As the most-studied clinically applied vehicles, liposomes faced with rapid recognition and clearance by immune system, limiting the efficacy on inflammatory disease. Existing synthetic nanoparticles are more likely to be rapidly eliminated from the circulation by the immune system [140]. However, polyethylene glycol has been applied to modified synthetic nanocarriers. The strategy prolongs half-life to days in the circulation, but the intrinsic character of the carrier is not changed. Naturally occurring EVs in the body may be subjected to less immune clearance [141]. EVs can undergo surface modification and drug loading through the cell metabolic process, which is advantageous for unstable biodrugs, such as RNAs or proteins. Both nanomedicines and EVs can reduce drug toxicity compared with free drugs. This may be related to enhanced targeted distribution of drugs and the reduction of toxic solvents used to dissolve insoluble drugs. Most nanomedicines on the market are liposome-related nanocarriers that can load up to two therapeutic substances and contain four phospholipid components [142]. However, liposomes can carry a wide variety of exogenous drugs, such as small molecules, peptides, and nucleic acids [140]. EVs are natural nanoparticles released by eukaryotic cells, similar in size and structure to liposomes. They can carry complex components, such as lipids, proteins, carbohydrates, and internal contents [142]. These therapeutic contents may exert more comprehensive effects to injured tissue. Blood or plasma contains abundant EVs, and the transfusion causes less adverse immune reactions [143]. Therefore, the intravenous administration of EVs may pose a reduced safety risk. Although EVs have not received clinical approval, existing studies generally conclude EVs are safe [39, 40, 144]. More research and clinic trials are required to prove the safety of EV therapy.
It is unclear how EV biogenesis, cellular origin, and composition affect pharmacokinetics. Systemically injected EVs may also lead to rapid clearance due to drug loading and modification in vitro [145]. Clinical trials focusing on administration methods are required and may reveal the mechanisms of immune clearance. Additionally, approved nanomedicines may cause hypersensitivity reactions and other safety concerns [146, 147]. However, the internal cargo of allogeneic EVs may elicit immune reactions, which will challenge the clinical application of EVs. The highly heterogeneous characteristics of EVs make purification and isolation important. Furthermore, EV production is associated with more biological variability. Both cell source and culture medium need to be controlled to obtain stable EVs [142]. EVs also require more stringent storage conditions than normal nanocarriers. Suitable protective agents and storage at −80 °C are essential for EVs preservation [144].
Overall, synthetic nanocarriers have the advantages of convenient synthesis, storage, and a higher possibility of obtaining clinical approval. However, EVs may exhibit better immune escape, have more complex structures and internal substances, and precise targeting effects. After solving large-scale production, storage, and other obstacles, EVs may become potential biodrugs for therapeutic therapy. Several challenges limit the potential clinical application of MSC-EVs as biodrugs. One obstacle is the poor yield of EVs generated by MSCs [108]. A dose of EVs for a 70-kg individual is produced from 7 billion MSCs, and during the expansion in vitro, the secretion of EVs is significantly reduced by MSC senescence [148, 149]. MSC bioreactors may entail high production costs [148, 149]. Furthermore, the potential tumorigenicity of MSC-EVs may be a disadvantage [150, 151].
In summary, ARDS has become a major public health challenge due to the high morbidity and lack of effective treatments. MSC-EVs may be used as biodrugs to treat ARDS because of their anti-inflammatory, cell injury repair, alveolar fluid clearance, and anti-microbial properties. Compared with other nanodrugs, MSC-EVs inherit the unique immune escape and inflammatory targeting characteristics of MSCs. The cargos endow MSC-EVs with more diverse regulatory and therapeutic abilities. MSC-EVs may be widely applied once large-scale production, storage, and biocompatibility issues are resolved. In addition, MSC-EVs can be combined with nanoparticles to achieve advantages of both and give MSC-EVs broader development prospects. Despite the current challenges in the clinical translation of MSC-EVs, several strategies have been developed to enhance their therapeutic effects, including genetic modification, preconditioning, and exogenous drug loading. Rapid development in this area will help to overcome challenges regarding the application of MSC-EVs as biodrugs to treat ARDS.
References
Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398(10300):622–37.
Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–33.
Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–54.
Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800.
Maca J, Jor O, Holub M, et al. Past and present ARDS mortality rates: a systematic review. Respir Care. 2017;62(1):113–22.
Pham T, Rubenfeld GD. Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome. A 50th birthday review. Am J Respir Crit Care Med. 2017;195(7):860–70.
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Tang X, Du RH, Wang R, et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158(1):195–205.
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361(26):2507–17.
Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–34.
Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2.
Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383(2):120–8.
Mauad T, Hajjar LA, Callegari GD, et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med. 2010;181(1):72–9.
Gupta E, Jacobs MD, George G, Roman J. Beyond the ICU: Frailty and post-ICU disability. Healthcare use after acute respiratory distress syndrome and severe sepsis. Am J Respir Crit Care Med. 2019;199(8):1028–30.
Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–23.
Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–15.
Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–86.
Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. In: Abbas AK, Galli SJ, Howley PM editors Annual Review of Pathology: Mechanisms of Disease. Vol 6. 147–163 (2011).
Lemyze M, Mallat J, Thevenin D. Prone positioning in the acute respiratory distress syndrome. N Engl J Med. 2013;369(10):980.
Curley GF, Laffey JG, Zhang H, Slutsky AS. Biotrauma and ventilator-induced lung injury: clinical implications. Chest. 2016;150(5):1109–17.
Albert RK, Smith B, Perlman CE, Schwartz DA. Is progression of pulmonary fibrosis due to ventilation-induced lung injury? Am J Respir Crit Care Med. 2019;200(2):140–51.
Tieu A, Hu K, Gnyra C, et al. Mesenchymal stromal cell extracellular vesicles as therapy for acute and chronic respiratory diseases: a meta-analysis. J Extracell Vesicles. 2021;10(12): e12141.
Monsel A, Zhu YG, Gennai S, Hao Q, Liu J, Lee JW. Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology. 2014;121(5):1099–121.
Abraham A, Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9(1):28–38.
Deng H, Wu L, Liu M, et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate LPS-induced ARDS by modulating macrophage polarization through inhibiting glycolysis in macrophages. Shock. 2020;54(6):828–43.
Hao Q, Gudapati V, Monsel A, et al. Mesenchymal stem cell-derived extracellular vesicles decrease lung injury in mice. J Immunol. 2019;203(7):1961–72.
Zhang GY, Liao T, Zhou SB, Fu XB, Li QF. Mesenchymal stem (stromal) cells for treatment of acute respiratory distress syndrome. Lancet Respir Med. 2015;3(4):e11-12.
Carnino JM, Hao Kwok Z, Jin Y. Extracellular vesicles: a novel opportunity for precision medicine in respiratory diseases. Front Med Lausanne. 2021;8:661679.
Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403.
Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85(1):3–10.
Lopes-Pacheco M, Robba C, Rocco PRM, Pelosi P. Current understanding of the therapeutic benefits of mesenchymal stem cells in acute respiratory distress syndrome. Cell Biol Toxicol. 2020;36(1):83–102.
Xiao K, He W, Guan W, et al. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-kappaB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020;11(10):863.
Xu Y, Zhu J, Feng B, et al. Immunosuppressive effect of mesenchymal stem cells on lung and gut CD8(+) T cells in lipopolysaccharide-induced acute lung injury in mice. Cell Prolif. 2021;54(5): e13028.
Periera-Simon S, Xia X, Catanuto P, et al. Anti-fibrotic effects of different sources of MSC in bleomycin-induced lung fibrosis in C57BL6 male mice. Respirology. 2021;26(2):161–70.
Wang NF, Bai CX. Bone marrow-derived mesenchymal stem cells modulate autophagy in RAW264.7 macrophages via the phosphoinositide 3-kinase/protein kinase B/heme oxygenase-1 signaling pathway under oxygen-glucose deprivation/restoration conditions. Chin Med J (Engl). 2021;134(6):699–707.
Huang T, Zhang T, Jiang X, et al. Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy. Sci Adv. 2021;7(40):eabj0534.
Ikonomou L, Wagner DE, Turner L, Weiss DJ. Translating basic research into safe and effective cell-based treatments for respiratory diseases. Ann Am Thorac Soc. 2019;16(6):657–68.
Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–62.
Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660–73.
Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–96.
Zhu F, Wang J, Qiu X, Li J, Xia Z. Smoke inhalation injury repaired by a bone marrow-derived mesenchymal stem cell paracrine mechanism: angiogenesis involving the Notch signaling pathway. J Trauma Acute Care Surg. 2015;78(3):565–72.
Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182(8):1047–57.
Leeman KT, Pessina P, Lee JH, Kim CF. Mesenchymal stem cells increase alveolar differentiation in lung progenitor organoid cultures. Sci Rep. 2019;9(1):6479.
Chang YS, Oh W, Choi SJ, et al. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18(8):869–86.
Zhen G, Liu H, Gu N, Zhang H, Xu Y, Zhang Z. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci. 2008;13:3415–22.
Huang K, Kang X, Wang X, et al. Conversion of bone marrow mesenchymal stem cells into type II alveolar epithelial cells reduces pulmonary fibrosis by decreasing oxidative stress in rats. Mol Med Rep. 2015;11(3):1685–92.
Cai C, Hou L, Zhang J, et al. The inhibitory effect of mesenchymal stem cells with rAd-NK4 on liver cancer. Appl Biochem Biotechnol. 2017;183(1):444–59.
Mojsilovic S, Jaukovic A, Kukolj T, et al. Tumorigenic aspects of MSC senescence-implication in cancer development and therapy. J Pers Med. 2021;11(11):1133.
Lee HY, Hong IS. Double-edged sword of mesenchymal stem cells: Cancer-promoting versus therapeutic potential. Cancer Sci. 2017;108(10):1939–46.
Romieu-Mourez R, Francois M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol. 2007;179(3):1549–58.
Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106(13):4057–65.
Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–19.
Togel FE, Westenfelder C. Mesenchymal stem cells: a new therapeutic tool for AKI. Nat Rev Nephrol. 2010;6(3):179–83.
Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297(5590):2256–9.
Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31-42.
Monsel A, Zhu YG, Gudapati V, Lim H, Lee JW. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin Biol Ther. 2016;16(7):859–71.
Timmers L, Lim SK, Hoefer IE, et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011;6(3):206–14.
Alessio N, Ozcan S, Tatsumi K, et al. The secretome of MUSE cells contains factors that may play a role in regulation of stemness, apoptosis and immunomodulation. Cell Cycle. 2017;16(1):33–44.
Kolosa K, Motaln H, Herold-Mende C, Korsic M, Lah TT. Paracrine effects of mesenchymal stem cells induce senescence and differentiation of glioblastoma stem-like cells. Cell Transplant. 2015;24(4):631–44.
Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2(1):20389.
Kalluri R, Lebleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):6977.
Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118(4):1917–50.
Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51.
Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44(11):2060–4.
Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472.
Tang XD, Shi L, Monsel A, et al. Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang-1 mRNA. Stem Cells. 2017;35(7):1849–59.
Sarkar A, Mitra S, Mehta S, Raices R, Wewers MD. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS ONE. 2009;4(9): e7140.
Wang N, Chen C, Yang D, et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):2085–92.
Yu M, Liu W, Li J, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11(1):350.
Carre JE, Orban JC, Re L, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182(6):745–51.
Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–65.
Agrawal A, Mabalirajan U. Rejuvenating cellular respiration for optimizing respiratory function: targeting mitochondria. Am J Physiol Lung Cell Mol Physiol. 2016;310(2):L103-113.
Antunes MA, Abreu SC, Cruz FF, et al. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res. 2014;15:118.
Jiang XC, Zhang T, Gao JQ. The in vivo fate and targeting engineering of crossover vesicle-based gene delivery system. Adv Drug Deliv Rev. 2022;187: 114324.
Kang M, Jordan V, Blenkiron C, Chamley LW. Biodistribution of extracellular vesicles following administration into animals: a systematic review. J Extracell Vesicles. 2021;10(8): e12085.
Zhou X, Li Z, Qi M, et al. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics. 2020;10(18):8197–210.
Imai T, Takahashi Y, Nishikawa M, et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015;4:26238.
Wiklander OPB, Nordin JZ, O’loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316–26316.
Xu Q, Zhang Z, Zhao L, et al. Tropism-facilitated delivery of CRISPR/Cas9 system with chimeric antigen receptor-extracellular vesicles against B-cell malignancies. J Control Release. 2020;326:455–67.
Zu M, Xie D, Canup BSB, et al. “Green” nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials. 2021;279: 121178.
Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–79.
Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30.
Shi MM, Yang QY, Monsel A, et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J Extracell Vesicles. 2021;10(10):12134.
Zhang D, Lee H, Wang X, Rai A, Groot M, Jin Y. Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol Ther. 2018;26(9):2119–30.
Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260.
Liu A, Zhang X, He H, et al. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin Biol Ther. 2020;20(2):125–40.
Park KS, Svennerholm K, Shelke GV, et al. Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem Cell Res Ther. 2019;10(1):231.
Varkouhi AK, He X, Teixeira Monteiro AP, et al. Immunophenotypic characterization and therapeutics effects of human bone marrow- and umbilical cord-derived mesenchymal stromal cells in an experimental model of sepsis. Exp Cell Res. 2021;399(2): 112473.
Nakao Y, Fukuda T, Zhang Q, et al. Exosomes from TNF-alpha-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306–24.
Zhao M, Liu S, Wang C, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA. ACS Nano. 2021;15(1):1519–38.
He J, Zhang N, Zhu Y, Jin R, Wu F. MSC spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through PI3K-Akt signaling pathway. Biomaterials. 2021;265: 120448.
Feng B, Zhu J, Xu Y, et al. Immunosuppressive effects of mesenchymal stem cells on lung B cell gene expression in LPS-induced acute lung injury. Stem Cell Res Ther. 2020;11(1):418.
Di Trapani M, Bassi G, Midolo M, et al. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep. 2016;6:24120.
Chen W, Huang Y, Han J, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64(4):831–40.
Lo Sicco C, Reverberi D, Balbi C, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med. 2017;6(3):1018–28.
Bull TM, Clark B, Mcfann K, Moss M. National Institutes of Health/National Heart L, Blood Institute AN. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2010;182(9):1123–8.
Hu S, Park J, Liu A, et al. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl Med. 2018;7(8):615–24.
Dutra Silva J, Su Y, Calfee CS, et al. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J. 2021;58:1.
Zhou Y, Xu H, Xu W, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4(2):34.
Monsel A, Zhu YG, Gennai S, et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192(3):324–36.
Sun Y, Shi H, Yin S, et al. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving beta-cell destruction. ACS Nano. 2018;12(8):7613–28.
Zhang L, Li Y, Guan CY, et al. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Res Ther. 2018;9(1):36.
Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9):1852.
Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1376–83.
Gennai S, Monsel A, Hao Q, Park J, Matthay MA, Lee JW. Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Transplant. 2015;15(9):2404–12.
Loy H, Kuok DIT, Hui KPY, et al. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A(H5N1) virus-associated acute lung injury. J Infect Dis. 2019;219(2):186–96.
Park J, Kim S, Lim H, et al. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019;74(1):43–50.
Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74.
Zhou P, Yang X, Wang X, et al. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;588(7836):E6.
Kumar V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front Immunol. 2020;11:1722.
Zheng G, Huang R, Qiu G, et al. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018;374(1):1–15.
Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28(12):2229–38.
Gupta N, Krasnodembskaya A, Kapetanaki M, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67(6):533–9.
Sung DK, Chang YS, Sung SI, Yoo HS, Ahn SY, Park WS. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta- defensin- 2 via toll- like receptor 4 signalling. Cell Microbiol. 2016;18(3):424–36.
Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ, Mcdermott AM. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res. 2005;30(5):385–94.
Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125(1):108–15.
Sacar Demirci MD, Adan A. Computational analysis of microRNA-mediated interactions in SARS-CoV-2 infection. PeerJ. 2020;8: e9369.
Zipkin M. Exosome redux. Nat Biotechnol. 2019;37(12):1395–400.
Varkouhi AK, Jerkic M, Ormesher L, et al. Extracellular vesicles from interferon-gamma-primed human umbilical cord mesenchymal stromal cells reduce escherichia coli-induced acute lung injury in rats. Anesthesiology. 2019;130(5):778–90.
Bourgine P, Le Magnen C, Pigeot S, Geurts J, Scherberich A, Martin I. Combination of immortalization and inducible death strategies to generate a human mesenchymal stromal cell line with controlled survival. Stem Cell Res. 2014;12(2):584–98.
Skarn M, Noordhuis P, Wang MY, et al. Generation and characterization of an immortalized human mesenchymal stromal cell line. Stem Cells Dev. 2014;23(19):2377–89.
Piffoux M, Volatron J, Cherukula K, et al. Engineering and loading therapeutic extracellular vesicles for clinical translation: a data reporting frame for comparability. Adv Drug Deliv Rev. 2021;178: 113972.
Kim HY, Kumar H, Jo MJ, et al. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett. 2018;18(8):4965–75.
Silva AK, Luciani N, Gazeau F, et al. Combining magnetic nanoparticles with cell derived microvesicles for drug loading and targeting. Nanomedicine. 2015;11(3):645–55.
Lu X, Han J, Xu X, et al. PGE2 promotes the migration of mesenchymal stem cells through the activation of FAK and ERK1/2 pathway. Stem Cells Int. 2017;2017:8178643.
Han J, Lu X, Zou L, Xu X, Qiu H. E-prostanoid 2 receptor overexpression promotes mesenchymal stem cell attenuated lung injury. Hum Gene Ther. 2016;27(8):621–30.
Chen X, Wu S, Tang L, et al. Mesenchymal stem cells overexpressing heme oxygenase-1 ameliorate lipopolysaccharide-induced acute lung injury in rats. J Cell Physiol. 2019;234(5):7301–19.
He H, Liu L, Chen Q, et al. Mesenchymal stem cells overexpressing angiotensin-converting enzyme 2 rescue lipopolysaccharide-induced lung injury. Cell Transplant. 2015;24(9):1699–715.
Toledano Furman NE, Lupu-Haber Y, Bronshtein T, et al. Reconstructed stem cell nanoghosts: a natural tumor targeting platform. Nano Lett. 2013;13(7):3248–55.
Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–710.
Gong C, Tian J, Wang Z, et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J Nanobiotechnol. 2019;17(1):93.
Kaneti L, Bronshtein T, Malkah Dayan N, et al. Nanoghosts as a novel natural nonviral gene delivery platform safely targeting multiple cancers. Nano Lett. 2016;16(3):1574–82.
Pomatto MAC, Bussolati B, D’antico S, et al. Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol Ther Methods Clin Dev. 2019;13:133–44.
Ge L, Xun C, Li W, et al. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J Nanobiotechnol. 2021;19(1):380.
Duan A, Shen K, Li B, et al. Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell Res Ther. 2021;12(1):427.
Wu D, Chang X, Tian J, et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J Nanobiotechnol. 2021;19(1):209.
Yang Z, Shi J, Xie J, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4(1):69–83.
Hu C, Zhao L, Zhang L, Bao Q, Li L. Mesenchymal stem cell-based cell-free strategies: safe and effective treatments for liver injury. Stem Cell Res Ther. 2020;11(1):377.
Wolfram J, Ferrari M. Clinical cancer nanomedicine. Nano Today. 2019;25:85–98.
Luo W, Dai Y, Chen Z, Yue X, Andrade-Powell KC, Chang J. Spatial and temporal tracking of cardiac exosomes in mouse using a nano-luciferase-CD63 fusion protein. Commun Biol. 2020;3(1):114.
Witwer KW, Wolfram J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat Rev Mater. 2021;6(2):103–6.
Busatto S, Walker SA, Grayson W, et al. Lipoprotein-based drug delivery. Adv Drug Deliv Rev. 2020;159:377–90.
Elsharkasy OM, Nordin JZ, Hagey DW, et al. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–43.
Walker S, Busatto S, Pham A, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9(26):8001–17.
Moghimi SM, Andersen AJ, Hashemi SH, et al. Complement activation cascade triggered by PEG-PL engineered nanomedicines and carbon nanotubes: the challenges ahead. J Control Release. 2010;146(2):175–81.
Chanan-Khan A, Szebeni J, Savay S, et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol. 2003;14(9):1430–7.
Hupfeld J, Gorr IH, Schwald C, et al. Modulation of mesenchymal stromal cell characteristics by microcarrier culture in bioreactors. Biotechnol Bioeng. 2014;111(11):2290–302.
Bustos ML, Huleihel L, Kapetanaki MG, et al. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med. 2014;189(7):787–98.
Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123(4):1542–55.
Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by National Natural Science Foundation of China (81703423) and the Fundamental Research Funds for the Central Universities (226-2022-00125).
Conflict of interest
The authors declare that they have no competing financial interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Not applicable.
Authors’ contributions
HS: Writing—original draft. TZ: Writing—review and editing. JG: Conceptualization and supervision.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, H., Zhang, T. & Gao, J. Extracellular Vesicles Derived from Mesenchymal Stem Cells: A Potential Biodrug for Acute Respiratory Distress Syndrome Treatment. BioDrugs 36, 701–715 (2022). https://doi.org/10.1007/s40259-022-00555-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00555-5