Abstract
Background
The increasing numbers of elderly patients and rising incidence of maculopathy raise concerns over arterial thromboembolic events (ATEs) with the use of intravitreal anti-vascular endothelial growth factor (VEGF) medications.
Objectives
This study aimed to compare the risk of ATEs between aflibercept and ranibizumab for maculopathy.
Methods
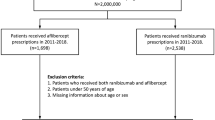
We conducted a retrospective population-based cohort study analyzing Taiwan’s National Health Insurance Database during 2011–2017 to identify patients with maculopathy receiving intravitreal aflibercept or ranibizumab. The primary outcome was any hospitalization or emergency room visit because of ATEs, including ischemic heart disease (IHD), ischemic stroke (IS), and transient ischemic attack (TIA). The secondary outcome was mortality within 30 days after occurrence of ATE. We employed propensity score methods to generate more homogeneous groups for comparison.
Results
We included 5791 aflibercept users and 14,534 ranibizumab users in this study. Compared with the ranibizumab group, the aflibercept group was associated with a lower risk of ATE (hazard ratio [HR] 0.85; 95% confidence interval [CI] 0.80–0.91), with HRs of 0.86 for IHD (95% CI 0.80–0.93), 0.87 for IS (95% CI 0.76–1.00), and 0.57 for TIA (95% CI 0.46–0.71). The risk of 30-day mortality after ATE (HR 1.39; 95% CI 0.80–2.43) and the risk of all-cause mortality (HR 1.02; 95% CI 0.89–1.17) in the aflibercept group was similar to that in the ranibizumab group.
Conclusion
The use of aflibercept in patients with maculopathy was associated with a lower risk of ATE than was the use of ranibizumab. There was no difference in mortality risk between the two groups. Our study could provide strong grounds for future prospective studies to confirm the findings.



Similar content being viewed by others
References
Sarwar S, Clearfield E, Soliman MK, Sadiq MA, Baldwin AJ, Hanout M, Agarwal A, Sepah YJ, Do DV, Nguyen QD. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2016;2:CD011346.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Wecker T, Ehlken C, Bühler A, Lange C, Agostini H, Böhringer D, Stahl A. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for amd, dme, rvo and myopic cnv. Br J Ophthalmol. 2017;101:353–9.
Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, Couvillion S, Nasir MA, Rabena MD, Maia M, Van Everen S, Le K, Hanley WD. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina (Philadelphia, Pa). 2017;37:1847–58.
Christoforidis JB, Briley K, Binzel K, Bhatia P, Wei L, Kumar K, Knopp MV. Systemic biodistribution and intravitreal pharmacokinetic properties of bevacizumab, ranibizumab, and aflibercept in a nonhuman primate model. Invest Ophthalmol Vis Sci. 2017;58:5636–45.
Thulliez M, Angoulvant D, Le Lez ML, Jonville-Bera AP, Pisella PJ, Gueyffier F, Bejan-Angoulvant T. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and meta-analysis. JAMA Ophthalmol. 2014;132:1317–26.
Rosenfeld PJ, Rich RM, Lalwani GA. Ranibizumab: phase iii clinical trial results. Ophthalmol Clin North Am. 2006;19:361–72.
García-Layana A, Figueroa MS, Araiz J, Ruiz-Moreno JM, Gómez-Ulla F, Arias-Barquet L, Reiter N. Treatment of exudative age-related macular degeneration: focus on aflibercept. Drugs Aging. 2015;32:797–807.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U. Intravitreal aflibercept (vegf trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, Couvillion S, Nasir MA, Rabena MD, Le K, Maia M, Visich JE. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular amd. Br J Ophthalmol. 2014;98:1636–41.
Maloney MH, Payne SR, Herrin J, Sangaralingham LR, Shah ND, Barkmeier AJ. Risk of systemic adverse events after intravitreal bevacizumab, ranibizumab, and aflibercept in routine clinical practice. Ophthalmology. 2021;128:417–24.
Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, Lai EC. Taiwan’s national health insurance research database: past and future. Clin Epidemiol. 2019;11:349–58.
Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the national health insurance research database with ischemic stroke cases in taiwan. Pharmacoepidemiol Drug Saf. 2011;20:236–42.
Cheng C-L, Lee C-H, Chen P-S, Li Y-H, Lin S-J, Yang Y-HK. Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J Epidemiol. 2014;24:500–7.
Xu Y, Tan CS. Safety and complications of intravitreal injections performed in an asian population in singapore. Int Ophthalmol. 2017;37:325–32.
Heier JS, Bressler NM, Avery RL, Bakri SJ, Boyer DS, Brown DM, Dugel PU, Freund KB, Glassman AR, Kim JE, Martin DF, Pollack JS, Regillo CD, Rosenfeld PJ, Schachat AP, Wells JA 3rd. Comparison of aflibercept, bevacizumab, and ranibizumab for treatment of diabetic macular edema: Extrapolation of data to clinical practice. JAMA Ophthalmol. 2016;134:95–9.
Lee WA, Cheng CL, Lee CH, Kao Yang YH. Risks of newly onset hemorrhagic stroke in patients with neovascular age-related macular degeneration. Pharmacoepidemiol Drug Saf. 2017;26:1277–85.
Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–11.
Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res. 2017;26:1654–70.
Avery RL, Gordon GM. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol. 2016;134:21–9.
Dugel PU, Singh N, Francom S, Cantrell RA, Grzeschik SM, Fung AE. The systemic safety of ranibizumab in patients 85 years and older with neovascular age-related macular degeneration. Ophthalmol Retina. 2018;2:667–75.
Zarbin MA, Francom S, Grzeschik S, Tuomi L, Haskova Z, Macfadden W, Margaron P, Snow H, Cruess A, Staurenghi G, Dunger-Baldauf C. Systemic safety in ranibizumab-treated patients with neovascular age-related macular degeneration: a patient-level pooled analysis. Ophthalmol Retina. 2018;2:1087–96.
Plyukhova AA, Budzinskaya MV, Starostin KM. Comparative safety of bevacizumab, ranibizumab, and aflibercept for treatment of neovascular age-related macular degeneration (amd): a systematic review and network meta-analysis of direct comparative studies. J Clin Med. 2020;9:1–14.
Pham B, Thomas SM, Lillie E, Lee T, Hamid J, Richter T, Janoudi G, Agarwal A, Sharpe JP, Scott A, Warren R, Brahmbhatt R, Macdonald E, Straus SE, Tricco AC. Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis. BMJ Open. 2019;9:1–11.
Rim TH, Yoo TK, Kwak J, Lee JS, Kim SH, Kim DW, Kim SS. Long-term regular use of low-dose aspirin and neovascular age-related macular degeneration. Ophthalmology. 2018;126:274–82.
Kim J, Kim DW, Kim DH, Ryu SY. Risk of stroke associated with intravitreal ranibizumab injections in age-related macular degeneration: a nationwide case-crossover study. Eye (Lond). 2020;35:601–7.
Rim TH, Lee CS, Lee SC, Kim DW, Kim SS. Intravitreal ranibizumab therapy for neovascular age-related macular degeneration and the risk of stroke: A national sample cohort study. Retina (Philadelphia, Pa). 2016;36:2166–74.
Jeon HL, Byun SJ, Pratt NL, Sultana J, Park SJ. Cardiovascular risk in patients receiving ranibizumab for exudative age-related macular degeneration: a nationwide self-controlled case-series study. Br J Ophthalmol. 2020;105:543–8.
Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal vegf trap. Br J Ophthalmol. 2008;92:667–8.
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ. Binding and neutralization of vascular endothelial growth factor (vegf) and related ligands by vegf trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–85.
García-Quintanilla L, Luaces-Rodríguez A. Pharmacokinetics of intravitreal anti-vegf drugs in age-related macular degeneration. Pharmaceutics. 2019;11:365.
Reibaldi M, Fallico M, Avitabile T, Bonfiglio V, Russo A, Castellino N, Parisi G, Longo A, Pulvirenti A, Boscia F, Virgili G. Risk of death associated with intravitreal anti-vascular endothelial growth factor therapy: a systematic review and meta-analysis. JAMA Ophthalmol. 2020;138:50–7.
Acknowledgement
The authors thank the Health Data Science Center, National Cheng Kung University Hospital for providing administrative support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Taiwan Ministry of Science and Technology (107-2320-B-006-070-MY3). The funding source had no role in the design, analysis, interpretation, or reporting of results, or in the decision to submit the manuscript for publication.
Conflict of interest
Wan-Ju Annabelle Lee, Shih-Chieh Shao, Tzu-Chi Liao, Swu-Jane Lin, Chi-Chun Lai, and Edward Chia-Cheng Lai have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
This study was approved by the Institutional Review Board of National Cheng Kung University Hospital (A-ER-106-501).
Consent
Not applicable.
Availability of Data and Material
Data sharing is not applicable because data analysis and management were only permitted within Taiwan’s Health and Welfare Data Science Center because of data safety and privacy concerns.
Code availability
Not applicable.
Author Contributions
WJAL and ECCL substantially contributed to the study conception and design. TCL substantially contributed to the data acquisition and analysis. All authors substantially contributed to the data interpretation and manuscript writing and gave final approval of the manuscript submission. ECCL is the guarantor for the whole of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, WJ.A., Shao, SC., Liao, TC. et al. Comparative Risk of Arterial Thromboembolic Events Between Aflibercept and Ranibizumab in Patients with Maculopathy: A Population-Based Retrospective Cohort Study. BioDrugs 35, 579–588 (2021). https://doi.org/10.1007/s40259-021-00497-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-021-00497-4