Abstract
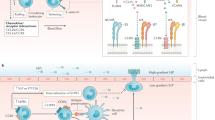
In the last two decades, understanding of inflammatory bowel disease (IBD) immunopathogenesis has expanded considerably. Histopathological examination of the intestinal mucosa in IBD demonstrates the presence of a chronic inflammatory cell infiltrate. Research has focused on identifying mechanisms of immune cell trafficking to the gastrointestinal tract that may represent effective gut-selective targets for IBD therapy whilst avoiding systemic immunosuppression that may be associated with off-target adverse effects such as infection and malignancy. Integrins are cell surface receptors that can bind to cellular adhesion molecules to mediate both leukocyte homing and retention. In 2014, Vedolizumab (Entyvio®) was the first anti-integrin (anti-α4ß7 monoclonal antibody) treatment to be approved for use in IBD. Several other anti-integrin therapies are currently in advanced stages of development, including novel orally administered small-molecule drugs. Drugs targeting alternative trafficking mechanisms such as mucosal addressin cellular adhesion molecule-1 and sphingosine-1-phosphate receptors are also being evaluated. Here, we summarise key established and emerging therapies targeting leukocyte trafficking that may play an important role in realising the goal of stratified precision medicine in IBD care.


Similar content being viewed by others
References
de Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13(1):13–27. https://doi.org/10.1038/nrgastro.2015.186.
Lamb CA, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Supplement_3):S1–106. https://doi.org/10.1136/gutjnl-2019-318484.
Dart RJ, et al. Results of the Seventh Scientific Workshop of ECCO: Precision Medicine in IBD—Challenges and Future Directions. J Crohns Colitis. 2021. https://doi.org/10.1093/ecco-jcc/jjab049.
Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009;31(3):389–400. https://doi.org/10.1016/j.immuni.2009.08.012.
Feakins RM. Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. J Clin Pathol. 2013;66(12):1005–26. https://doi.org/10.1136/jclinpath-2013-201885.
Brandtzaeg P, et al. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1(1):31–7. https://doi.org/10.1038/mi.2007.9.
von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–78. https://doi.org/10.1038/nri1222.
Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–89. https://doi.org/10.1038/nri2156.
Butcher EC. Leukocyte-endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell. 1991;67(6):1033–6. https://doi.org/10.1016/0092-8674(91)90279-8.
Simon SI, et al. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164(8):4348–58. https://doi.org/10.4049/jimmunol.164.8.4348.
da Costa Martins P, et al. P-selectin glycoprotein ligand-1 is expressed on endothelial cells and mediates monocyte adhesion to activated endothelium. Arterioscler Thrombos Vasc Biol. 2007;27(5):1023–9. https://doi.org/10.1161/ATVBAHA.107.140442.
Rivera-Nieves J, et al. Critical role of endothelial P-selectin glycoprotein ligand 1 in chronic murine ileitis. J Exp Med. 2006;203(4):907–17. https://doi.org/10.1084/jem.20052530.
Eriksson EE, et al. Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J Exp Med. 2001;194(2):205–18. https://doi.org/10.1084/jem.194.2.205.
Arbonés ML, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1(4):247–60. https://doi.org/10.1016/1074-7613(94)90076-0.
Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48(4):549–54. https://doi.org/10.1016/0092-8674(87)90233-9.
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. https://doi.org/10.1016/S0092-8674(02)00971-6.
Alberts B, et al. Cells in their social context: cell junctions and the extracellular, in matrix, molecular biology of the cell. New York: Garland Science; 2014. p. 1035–90.
Lamb CA, et al. Gut-selective integrin-targeted therapies for inflammatory bowel disease. J Crohn’s Colitis. 2018;12(Supplement_2):S653–68. https://doi.org/10.1093/ecco-jcc/jjy060.
Beglova N, et al. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat Struct Biol. 2002;9(4):282–7. https://doi.org/10.1038/nsb779.
Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11(6):416–26. https://doi.org/10.1038/nri2986.
Takagi J, et al. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110(5):599–611. https://doi.org/10.1016/S0092-8674(02)00935-2.
Springer TA, Wang J-H. The three-dimensional structure of integrins and their ligands, and conformational regulation of cell adhesion, in advances in protein chemistry. Academic Press; 2004. p. 29–63.
Shimaoka M, et al. Structures of the αL I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell. 2003;112(1):99–111. https://doi.org/10.1016/S0092-8674(02)01257-6.
Evans R, et al. The integrin LFA-1 signals through ZAP-70 to regulate expression of high-affinity LFA-1 on T lymphocytes. Blood. 2011;117(12):3331–42. https://doi.org/10.1182/blood-2010-06-289140.
Constantin G, et al. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13(6):759–69. https://doi.org/10.1016/s1074-7613(00)00074-1.
Carrasco YR, et al. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity. 2004;20(5):589–99. https://doi.org/10.1016/s1074-7613(04)00105-0.
Dustin ML. The cellular context of T cell signaling. Immunity. 2009;30(4):482–92. https://doi.org/10.1016/j.immuni.2009.03.010.
Briskin M, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151(1):97–110.
Erle DJ, et al. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153(2):517–28.
Rott LS, et al. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with beta 7 integrins and memory differentiation. J Immunol. 1996;156(10):3727–36.
Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med. 2009;9(7):836–50. https://doi.org/10.2174/156652409789105525.
Newham P, et al. Alpha4 integrin binding interfaces on VCAM-1 and MAdCAM-1. Integrin binding footprints identify accessory binding sites that play a role in integrin specificity. J Biol Chem. 1997;272(31):19429–40. https://doi.org/10.1074/jbc.272.31.19429.
Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–38. https://doi.org/10.1016/j.immuni.2004.08.011.
Kunkel EJ, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192(5):761–8. https://doi.org/10.1084/jem.192.5.761.
Papadakis KA, et al. CCR9-Positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn’s disease. Gastroenterology. 2001;121(2):246–54. https://doi.org/10.1053/gast.2001.27154.
Cepek KL, et al. Integrin alpha E beta 7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150(8):3459.
Kilshaw PJ. Alpha E beta 7. Mol Pathol. 1999;52(4):203–7. https://doi.org/10.1136/mp.52.4.203.
Schön MP, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162(11):6641–9.
Taraszka KS, et al. Molecular basis for leukocyte integrin alpha(E)beta(7) adhesion to epithelial (E)-cadherin. J Exp Med. 2000;191(9):1555–67. https://doi.org/10.1084/jem.191.9.1555.
Cerf-Bensussan N, et al. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987;17(9):1279–85. https://doi.org/10.1002/eji.1830170910.
Higgins JM, et al. Direct and regulated interaction of integrin alphaEbeta7 with E-cadherin. J Cell Biol. 1998;140(1):197–210. https://doi.org/10.1083/jcb.140.1.197.
Sarnacki S, et al. Enhancement of CD3-induced activation of human intestinal intraepithelial lymphocytes by stimulation of the beta 7-containing integrin defined by HML-1 monoclonal antibody. Eur J Immunol. 1992;22(11):2887–92. https://doi.org/10.1002/eji.1830221120.
Gebhardt T, Mackay LK. Local immunity by tissue-resident CD8(+) memory T cells. Front Immunol. 2012;3:340–340. https://doi.org/10.3389/fimmu.2012.00340.
Lim SP, Leung E, Krissansen GW. The beta7 integrin gene (Itgb-7) promoter is responsive to TGF-beta1: defining control regions. Immunogenetics. 1998;48(3):184–95. https://doi.org/10.1007/s002510050422.
Robinson PW, et al. Studies on transcriptional regulation of the mucosal T-cell integrin αEβ7 (CD103). Immunology. 2001;103(2):146–54. https://doi.org/10.1046/j.1365-2567.2001.01232.x.
Lamb CA, et al. αEβ7 Integrin Identifies Subsets of Pro-Inflammatory Colonic CD4+ T Lymphocytes in Ulcerative Colitis. J Crohns Colitis. 2017;11(5):610–20. https://doi.org/10.1093/ecco-jcc/jjw189.
Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110(4):975–84. https://doi.org/10.1053/gast.1996.v110.pm8613031.
Ichikawa R, et al. AlphaE integrin expression is increased in the ileum relative to the colon and unaffected by inflammation. J Crohn’s Colitis. 2018;12(10):1191–9. https://doi.org/10.1093/ecco-jcc/jjy084.
Brandtzaeg P, et al. Lymphoepithelial interactions in the mucosal immune system. Gut. 1988;29(8):1116–30. https://doi.org/10.1136/gut.29.8.1116.
Gavin MA, et al. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat Immunol. 2002;3(1):33–41. https://doi.org/10.1038/ni743.
Zelenika D, et al. Regulatory T cells overexpress a subset of Th2 gene transcripts. J Immunol. 2002;168(3):1069–79. https://doi.org/10.4049/jimmunol.168.3.1069.
Lehmann J, et al. Expression of the integrin alpha E beta 7 identifies unique subsets of CD25+ as well as CD25-regulatory T cells. Proc Natl Acad Sci USA. 2002;99(20):13031–6. https://doi.org/10.1073/pnas.192162899.
Zhao D, et al. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112(5):2129–38. https://doi.org/10.1182/blood-2008-02-140277.
Huehn J, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199(3):303–13. https://doi.org/10.1084/jem.20031562.
Lúdvíksson BR, et al. Administration of mAb against alpha E beta 7 prevents and ameliorates immunization-induced colitis in IL-2-/- mice. J Immunol. 1999;162(8):4975–82.
Picarella D, et al. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158(5):2099–106.
Madara JL, et al. Characterization of spontaneous colitis in cotton-top tamarins (Saguinus oedipus) and its response to sulfasalazine. Gastroenterology. 1985;88(1 Pt 1):13–9. https://doi.org/10.1016/s0016-5085(85)80126-8.
Podolsky DK, et al. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J Clin Investig. 1993;92(1):372–80. https://doi.org/10.1172/jci116575.
Hesterberg PE, et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7. Gastroenterology. 1996;111(5):1373–80. https://doi.org/10.1053/gast.1996.v111.pm8898653.
Yednock TA, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356(6364):63–6. https://doi.org/10.1038/356063a0.
Kent SJ, et al. A monoclonal antibody to alpha 4 integrin suppresses and reverses active experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58(1):1–10. https://doi.org/10.1016/0165-5728(94)00165-k.
Engelhardt B, et al. The development of experimental autoimmune encephalomyelitis in the mouse requires alpha4-integrin but not alpha4beta7-integrin. J Clin Investig. 1998;102(12):2096–105. https://doi.org/10.1172/JCI4271.
Bauer M, et al. β integrins differentially control extravasation of inflammatory cell subsets into the CNS during autoimmunity. Proc Natl Acad Sci. 2009;106(6):1920. https://doi.org/10.1073/pnas.0808909106.
Léger OJ, et al. Humanization of a mouse antibody against human alpha-4 integrin: a potential therapeutic for the treatment of multiple sclerosis. Hum Antibodies. 1997;8(1):3–16.
Tubridy N, et al. The effect of anti-alpha4 integrin antibody on brain lesion activity in MS. The UK Antegren Study Group. Neurology. 1999;53(3):466–72. https://doi.org/10.1212/wnl.53.3.466.
Gordon FH, et al. A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha4 integrin in active Crohn’s disease. Gastroenterology. 2001;121(2):268–74. https://doi.org/10.1053/gast.2001.26260.
Gordon FH, et al. A pilot study of treatment of active ulcerative colitis with natalizumab, a humanized monoclonal antibody to alpha-4 integrin. Aliment Pharmacol Ther. 2002;16(4):699–705. https://doi.org/10.1046/j.1365-2036.2002.01205.x.
Sandborn WJ, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353(18):1912–25. https://doi.org/10.1056/NEJMoa043335.
Targan SR, et al. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2007;132(5):1672–83. https://doi.org/10.1053/j.gastro.2007.03.024.
Van Assche G, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353(4):362–8. https://doi.org/10.1056/NEJMoa051586.
Richardson EP. progressive multifocal leukoencephalopathy. N Engl J Med. 1961;265(17):815–23. https://doi.org/10.1056/nejm196110262651701.
Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425–37. https://doi.org/10.1016/s1474-4422(10)70040-5.
Miller, A. Assessing the Withdrawal of Natalizumab. Journal Watch 2005. https://www.jwatch.org/jn200507070000001/2005/07/07/assessing-withdrawal-natalizumab. Accessed 1 March 2021.
Langer-Gould A, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353(4):375–81. https://doi.org/10.1056/NEJMoa051847.
Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353(4):369–74. https://doi.org/10.1056/NEJMoa051782.
Huggett B. How Tysabri survived. Nat Biotechnol. 2009;27(11):986. https://doi.org/10.1038/nbt1109-986.
Biogen. FDA approves Tysabri® for the treatment of moderate-to-severe Crohn's Disease. 2008. https://investors.biogen.com/news-releases/news-release-details/fda-approves-tysabrir-treatment-moderate–severe-crohns-disease. Accessed 1 Mar 2021.
Giovannoni G, et al. Updated incidence of natalizumab-associated progressive multifocal leukoencephalopathy (PML) and its relationship with natalizumab exposure over time (2815). Neurology. 2020;94(Supplemebt_15):2815.
Soler D, et al. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330(3):864–75. https://doi.org/10.1124/jpet.109.153973.
Stefanich EG, et al. A humanized monoclonal antibody targeting the β7 integrin selectively blocks intestinal homing of T lymphocytes. Br J Pharmacol. 2011;162(8):1855–70. https://doi.org/10.1111/j.1476-5381.2011.01205.x.
Haanstra KG, et al. Antagonizing the α4β1 integrin, but not α4β7, inhibits leukocytic infiltration of the central nervous system in rhesus monkey experimental autoimmune encephalomyelitis. J Immunol. 2013;190(5):1961–73. https://doi.org/10.4049/jimmunol.1202490.
Döring A, et al. TET inducible expression of the α4β7-integrin ligand MAdCAM-1 on the blood–brain barrier does not influence the immunopathogenesis of experimental autoimmune encephalomyelitis. Eur J Immunol. 2011;41(3):813–21. https://doi.org/10.1002/eji.201040912.
Milch C, et al. Vedolizumab, a monoclonal antibody to the gut homing α4ß7 integrin, does not affect cerebrospinal fluid T-lymphocyte immunophenotype. J Neuroimmunol. 2013;264(1):123–6. https://doi.org/10.1016/j.jneuroim.2013.08.011.
Wyant T, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64(1):77–83. https://doi.org/10.1136/gutjnl-2014-307127.
Feagan BG, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. https://doi.org/10.1056/NEJMoa1215734.
Feagan BG, et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2017;15(2):229-239.e5. https://doi.org/10.1016/j.cgh.2016.08.044.
Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–21. https://doi.org/10.1056/NEJMoa1215739.
Sands BE, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147(3):618-627.e3. https://doi.org/10.1053/j.gastro.2014.05.008.
Sands BE, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381(13):1215–26. https://doi.org/10.1056/NEJMoa1905725.
Sandborn WJ, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257-265.e3. https://doi.org/10.1053/j.gastro.2011.10.032.
Colombel J-F, et al. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14(2):254–66. https://doi.org/10.1093/ecco-jcc/jjz131.
Turner D, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. https://doi.org/10.1053/j.gastro.2020.12.031.
Feagan BG, et al. Rapid response to vedolizumab therapy in biologic-naive patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17(1):130-138.e7. https://doi.org/10.1016/j.cgh.2018.05.026.
Faleck DM, et al. Shorter disease duration is associated with higher rates of response to vedolizumab in patients with crohn’s disease but not ulcerative colitis. Clin Gastroenterol Hepatol. 2019;17(12):2497-2505.e1. https://doi.org/10.1016/j.cgh.2018.12.040.
Zundler S, et al. Immune cell trafficking and retention in inflammatory bowel disease: mechanistic insights and therapeutic advances. Gut. 2019;68(9):1688–700. https://doi.org/10.1136/gutjnl-2018-317977.
Zundler S, et al. Blockade of αEβ7 integrin suppresses accumulation of CD8(+) and Th9 lymphocytes from patients with IBD in the inflamed gut in vivo. Gut. 2017;66(11):1936–48. https://doi.org/10.1136/gutjnl-2016-312439.
Kotze PG, et al. Real-world clinical, endoscopic and radiographic efficacy of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Therap. 2018;48(6):626–37. https://doi.org/10.1111/apt.14919.
Loftus EV Jr, et al. Long-term safety of vedolizumab for inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52(8):1353–65. https://doi.org/10.1111/apt.16060.
Takeda Pharmaceuticals. Safety Data for the Long Term. News Releases 2020. https://www.entyviohcp.com/safety-profile. Accessed 7 Apr 2021.
Mahadevan U, et al. Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease. Gastroenterology. 2021;160(4):1131–9. https://doi.org/10.1053/j.gastro.2020.11.038.
Verstockt B, et al. Expression levels of 4 genes in colon tissue might be used to predict which patients will enter endoscopic remission after vedolizumab therapy for inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18(5):1142-1151.e10. https://doi.org/10.1016/j.cgh.2019.08.030.
Ananthakrishnan AN, et al. Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe. 2017;21(5):603-610.e3. https://doi.org/10.1016/j.chom.2017.04.010.
Zeissig S, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut. 2019;68(1):25–39. https://doi.org/10.1136/gutjnl-2018-316023.
Vermeire S, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. The Lancet. 2014;384(9940):309–18. https://doi.org/10.1016/s0140-6736(14)60661-9.
Tew GW, et al. Association between response to etrolizumab and expression of integrin αE and granzyme A in colon biopsies of patients with ulcerative colitis. Gastroenterology. 2016;150(2):477-487.e9. https://doi.org/10.1053/j.gastro.2015.10.041.
Joeckel LT, Bird PI. Are all granzymes cytotoxic in vivo? Biol Chem. 2014;395(2):181–202. https://doi.org/10.1515/hsz-2013-0238.
Anthony DA, et al. Functional dissection of the granzyme family: cell death and inflammation. Immunol Rev. 2010;235(1):73–92. https://doi.org/10.1111/j.0105-2896.2010.00907.x.
Sandborn WJ, et al. Etrolizumab for the treatment of ulcerative colitis and Crohn’s disease: an overview of the phase 3 clinical program. Adv Ther. 2020;37(7):3417–31. https://doi.org/10.1007/s12325-020-01366-2.
Peyrin-Biroulet L, et al. Etrolizumab as induction and maintenance therapy in patients with ulcerative colitis previously exposed to anti-tumor necrosis factor agent: the randomized; phase 3 HICKORY trial. United Eur Gastroenterol J. 2020;8(10):1258–75. https://doi.org/10.1177/2050640620968709.
ClinicalTrials.gov. A study of the efficacy and safety of etrolizumab in participants with ulcerative colitis who have been previously exposed to tumor necrosis factor (TNF) inhibitors (HICKORY). 2014. https://clinicaltrials.gov/ct2/show/NCT02100696. Accessed 15 Mar 2021.
Dotan I, et al. Etrolizumab compared with adalimumab or placebo as induction therapy for ulcerative colitis: results from the randomized; phase 3 HIBISCUS I & II trials. United Eur Gastroenterol J. 2020;8(10):1258–75. https://doi.org/10.1177/2050640620968709.
Danese S, et al. Etrolizumab versus infliximab for treating patients with moderately to severely active ulcerative colitis: results from the phase 3 GARDENIA study. United Eur Gastroenterol J. 2020;8(10):1258–75. https://doi.org/10.1177/2050640620968709.
Vermeire S, et al. LB18 Etrolizumab versus placebo in tumor necrosis factor antagonist naive patients with ulcerative colitis: results from the randomized phase III LAUREL trial. United Eur Gastroenterol J. 2020;8(10):1258–75. https://doi.org/10.1177/2050640620968709.
ClinicalTrials.gov. A study to assess whether etrolizumab is a safe and efficacious treatment for participants with moderately to severely active Crohn's disease (BERGAMOT). 2015. https://clinicaltrials.gov/ct2/show/NCT02394028. Accessed 20 Mar 2021.
Selinger C, et al. OTU-003 Etrolizumab as induction therapy in moderate to severe crohn’s disease: results from Bergamot cohort 1. Gut. 2018;67(Supplement_1):A53. https://doi.org/10.1136/gutjnl-2018-BSGAbstracts.106.
Sandborn WJ, et al. Efficacy and Safety of Abrilumab in a Randomized; Placebo-Controlled Trial for Moderate-to-Severe Ulcerative Colitis. Gastroenterology. 2019;156(4):946-957.e18. https://doi.org/10.1053/j.gastro.2018.11.035.
ClinicalTrials.gov. Abrilumab (AMG 181) in adults with moderate to severe Crohn's disease. 2012. https://clinicaltrials.gov/ct2/show/NCT01696396. Accessed 22 Mar 2021.
Sandborn WJ, et al. OP035 Efficacy and safety of abrilumab (AMG 181/MEDI 7183) therapy for moderate to severe Crohn’s disease. J Crohn’s Colitis. 2017;11(Supplement_1):S22–3. https://doi.org/10.1093/ecco-jcc/jjx002.034.
Mattheakis L, et al. P-126 PTG-100, An Oral Peptide Antagonist of Integrin α4β7 that Alters Trafficking of Gut Homing T Cells in Preclinical Animal Models. Inflamm Bowel Dis. 2016;22:S48. https://doi.org/10.1097/01.MIB.0000480232.55276.b3.
ClinicalTrials.gov. Safety and efficacy study of PTG-100 in the treatment of moderate to severe ulcerative colitis. 2016. https://clinicaltrials.gov/ct2/show/NCT02895100. Accessed 30 Mar 2021.
Sandborn W, et al. PTG-100, an oral gut-restricted peptide α4β7 antagonist, induces clinical and histologic remission in patients with moderate to severely active ulcerative colitis. United Eur Gastroenterol J. 2018;6(10):1586–97. https://doi.org/10.1177/2050640618812015.
Mattheakis L, et al. 416 - The oral α4β7 integrin specific antagonist PN-10943 is more effective than PTG-100 in multiple preclinical studies. Gastroenterology. 2019;156(6):S80–1. https://doi.org/10.1016/S0016-5085(19)36988-4.
Gupta SK, et al. Safety, pharmacokinetics, and pharmacodynamics of the novel oral peptide therapeutic PN-10943 (alpha4beta7 integrin antagonist) in normal healthy volunteers. Am J Gastroenterol. 2019;114:S430–1. https://doi.org/10.14309/01.ajg.0000592456.89314.1e .
ClinicalTrials.gov. PN-943 in adults with moderate to severe active ulcerative colitis (UC). 2020. https://clinicaltrials.gov/ct2/show/NCT04504383. Accessed 30 Mar 2021.
Yoshimura N, et al. Safety and Efficacy of AJM300, an Oral Antagonist of α4 Integrin, in Induction Therapy for Patients With Active Ulcerative Colitis. Gastroenterology. 2015;149(7):1775-1783.e2. https://doi.org/10.1053/j.gastro.2015.08.044.
Sugiura T, et al. Oral treatment with a novel small molecule alpha 4 integrin antagonist, AJM300, prevents the development of experimental colitis in mice. Journal of Crohn’s and Colitis. 2013;7(11):e533-542. https://doi.org/10.1016/j.crohns.2013.03.014.
Stüve O, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59(5):743–7. https://doi.org/10.1002/ana.20858.
Bloomgren G, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366(20):1870–80. https://doi.org/10.1056/NEJMoa1107829.
ClinicalTrials.gov. A study to evaluate the safety and efficacy of AJM300 in participants with active ulcerative colitis. 2018. https://clinicaltrials.gov/ct2/show/record/NCT03531892. Accessed 22 Mar 2021.
EA Pharma Co Ltd. Results of Phase III Clinical Study of AJM300 (Nonproprietary Name: Carotegrast Methyl) for Treatment of Ulcerative Colitis Conducted in Japan (AJM300 ⁄ CT3 Study)–Primary Endpoint. https://www.eapharma.co.jp/en/news/2021/0113.html. Accessed 22 Mar 2021.
Takazoe M, et al. S1066 oral alpha-4 integrin inhibitor (AJM300) in patients with active Crohn’s disease—a randomized, double-blind; placebo-controlled trial. Gastroenterology. 2009;136(5):A181. https://doi.org/10.1016/S0016-5085(09)60816-7.
Eisai Co Ltd. Major R&D Pipeline. 2021. https://www.eisai.com/company/business/research/pdf/epipeline.pdf. Accessed 29 Mar 2021.
Ohkuro M, et al. Calreticulin and integrin alpha dissociation induces anti-inflammatory programming in animal models of inflammatory bowel disease. Nat Commun. 2018;9(1):1982. https://doi.org/10.1038/s41467-018-04420-4.
University of Tsukuba, EA Pharma Co Ltd, and Eisai Co Ltd. Potent new mechanism of action for treatment of inflammatory bowel disease revealed. 2018. https://www.eisai.com/news/2018/pdf/enews201840pdf.pdf. Accessed 29 Mar 2021.
ClinicalTrials.gov. Study of E6007 in Japanese patients with moderate active ulcerative colitis. 2017. https://clinicaltrials.gov/ct2/show/NCT03018054. Accessed 29 Mar 2021.
Danese S, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med. 2014;160(10):704–11. https://doi.org/10.7326/m13-2403.
Vickers AD, et al. Systematic review with network meta-analysis: comparative efficacy of biologics in the treatment of moderately to severely active ulcerative colitis. PLoS ONE. 2016;11(10): e0165435. https://doi.org/10.1371/journal.pone.0165435.
Sandborn WJ, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):562-572.e12. https://doi.org/10.1053/j.gastro.2019.08.027.
Pharmaceuticals, T. European Commission Approves Subcutaneous Formulation of Entyvio® (Vedolizumab) for use as Maintenance Therapy in Adults with Moderately to Severely Active Ulcerative Colitis or Crohn’s Disease. 2020. https://www.takeda.com/newsroom/newsreleases/2020/european-commission-approves-subcutaneous-entyvio-for-use-as-maintenance-therapy-in-ulcerative-colitis-or-crohns-disease/. Accessed 30 July 2021.
Kobayashi M, et al. GlcNAc6ST-1-mediated decoration of MAdCAM-1 protein with L-selectin ligand carbohydrates directs disease activity of ulcerative colitis. Inflamm Bowel Dis. 2009;15(5):697–706. https://doi.org/10.1002/ibd.20827.
Sikorski EE, et al. The Peyer’s patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-alpha and IL-1. J Immunol. 1993;151(10):5239–50.
Connor EM, et al. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol. 1999;65(3):349–55. https://doi.org/10.1002/jlb.65.3.349.
Arihiro S, et al. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn’s disease. Pathol Int. 2002;52(5–6):367–74. https://doi.org/10.1046/j.1440-1827.2002.01365.x.
Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer’s patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3(1):99–108. https://doi.org/10.1016/1074-7613(95)90162-0.
Pullen N, et al. Pharmacological characterization of PF-00547659, an anti-human MAdCAM monoclonal antibody. Br J Pharmacol. 2009;157(2):281–93. https://doi.org/10.1111/j.1476-5381.2009.00137.x.
Vermeire S, et al. The mucosal addressin cell adhesion molecule antibody PF-00547,659 in ulcerative colitis: a randomised study. Gut. 2011;60(8):1068–75. https://doi.org/10.1136/gut.2010.226548.
ClinicalTrials.gov. A study to investigate the safety and efficacy properties Of PF-00547659 in patients with active ulcerative colitis. 2009. https://clinicaltrials.gov/ct2/show/NCT00928681. Accessed 31 Mar 2021.
Vermeire S, et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. The Lancet. 2017;390(10090):135–44. https://doi.org/10.1016/S0140-6736(17)30930-3.
ClinicalTrials.gov. A study of PF-00547659 in patients with moderate to severe ulcerative colitis (TURANDOT). 2012. https://clinicaltrials.gov/ct2/show/NCT01620255. Accessed 31 March 2021.
Reinisch W, et al. Long-term safety and efficacy of the anti-MAdCAM-1 monoclonal antibody ontamalimab (SHP647) for the treatment of ulcerative colitis: the open-label study TURANDOT II. J Crohn’s Colitis. 2021. https://doi.org/10.1093/ecco-jcc/jjab023 (Epub ahead of print(18 Feb 2021)).
Sandborn WJ, et al. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: report of the OPERA study. Gut. 2018;67(10):1824–35. https://doi.org/10.1136/gutjnl-2016-313457.
Su C, et al. A meta-analysis of the placebo rates of remission and response in clinical trials of active Crohn’s disease. Gastroenterology. 2004;126(5):1257–69. https://doi.org/10.1053/j.gastro.2004.01.024.
Sandborn WJ, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367(16):1519–28. https://doi.org/10.1056/NEJMoa1203572.
Feagan BG, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6(12):1370–7. https://doi.org/10.1016/j.cgh.2008.06.007.
Hassan-Zahraee M, et al. Anti-madcam antibody increases ß7+ T cells and CCR9 gene expression in the peripheral blood of patients with Crohn’s disease. J Crohn’s Colitis. 2018;12(1):77–86. https://doi.org/10.1093/ecco-jcc/jjx121.
ClinicalTrials.gov. Long-term safety of PF-00547659 In ulcerative colitis (TURANDOT II). 2013. https://clinicaltrials.gov/ct2/show/NCT01771809. Accessed 1 Apr 2021.
D’Haens G, et al. Effect of PF-00547659 on central nervous system immune surveillance and circulating β7+ T cells in Crohn’s disease: report of the TOSCA study. J Crohns Colitis. 2017;12(2):188–96. https://doi.org/10.1093/ecco-jcc/jjx128.
ClinicalTrials.gov. Evaluate PF-00547659 on cerebrospinal fluid lymphocytes in volunteers with Crohn's disease or ulcerative colitis who failed or did not tolerate anti-TNFs (TOSCA). 2020. https://clinicaltrials.gov/ct2/show/NCT01387594. Accessed 1 Apr 2021.
ClinicalTrials.gov. A study to monitor long-term treatment with PF-00547659 (OPERA II). 2011. https://clinicaltrials.gov/ct2/show/NCT01298492. Accessed 1 Apr 2021.
D’Haens G, et al. P037 Long-term safety, efficacy and pharmacokinetics of the anti-Mucosal Addressin Cell Adhesion Molecule-1 (MAdCAM-1) monoclonal antibody SHP647 in Crohn’s disease: THe Opera II Study. Gastroenterology. 2019;156(Supplement_3):S26–7. https://doi.org/10.1053/j.gastro.2019.01.089.
European Commission, Commission Decision of 20/11/2018 declaring a concentration to be compatible with the common market (Case No COMP/M.8955 - TAKEDA / SHIRE) according to Council Regulation (EC) No 139/2004 2018, Office for Official Publications of the European Union: Brussels.
Takeda. European Commission Releases Takeda from Commitment to Divest Shire’s Pipeline Compound SHP647. News Releases 2020. https://www.takeda.com/newsroom/newsreleases/2020/european-commission-releases-takeda-from-commitment-to-divest--shires-pipeline-compound-shp647/. Accessed 2 Apr 2021.
ClinicalTrials.gov. A safety extension study of ontamalimab in participants with moderate to severe ulcerative colitis or Crohn's disease (AIDA). 2017. https://clinicaltrials.gov/ct2/show/NCT03283085. Accessed 2 Apr 2021.
Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5(7):560–70. https://doi.org/10.1038/nri1650.
O’Sullivan C, Dev KK. The structure and function of the S1P1 receptor. Trends Pharmacol Sci. 2013;34(7):401–12. https://doi.org/10.1016/j.tips.2013.05.002.
Memon RA, et al. Endotoxin and cytokines increase hepatic sphingolipid biosynthesis and produce lipoproteins enriched in ceramides and sphingomyelin. Arterioscler Thromb Vasc Biol. 1998;18(8):1257–65. https://doi.org/10.1161/01.atv.18.8.1257.
Lo CG, et al. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med. 2005;201(2):291–301. https://doi.org/10.1084/jem.20041509.
Chun J, Hartung H-P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33(2):91–101. https://doi.org/10.1097/WNF.0b013e3181cbf825.
Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–4. https://doi.org/10.1038/nature04606.
Swan DJ, Kirby JA, Ali S. Post-transplant immunosuppression: regulation of the efflux of allospecific effector T cells from lymphoid tissues. PLoS ONE. 2012;7(9): e45548. https://doi.org/10.1371/journal.pone.0045548.
Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309(5741):1735–9. https://doi.org/10.1126/science.1113640.
Brinkmann V, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277(24):21453–7. https://doi.org/10.1074/jbc.C200176200.
Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G protein-coupled receptors. Feder Am Soc Exp Biol J. 2004;18(3):551–3. https://doi.org/10.1096/fj.03-0910fje.
Weinreich MA, Hogquist KA. Thymic emigration: when and how T cells leave home. J Immunol. 2008;181(4):2265–70. https://doi.org/10.4049/jimmunol.181.4.2265.
Ledgerwood LG, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9(1):42–53. https://doi.org/10.1038/ni1534.
Fujii T, et al. FTY720 suppresses the development of colitis in lymphoid-null mice by modulating the trafficking of colitogenic CD4+ T cells in bone marrow. Eur J Immunol. 2008;38(12):3290–303. https://doi.org/10.1002/eji.200838359.
Fujii R, et al. FTY720 suppresses CD4+CD44highCD62L− effector memory T cell-mediated colitis. Am J Physiol Gastrointest Liver Physiol. 2006;291(2):G267–74. https://doi.org/10.1152/ajpgi.00496.2005.
Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115(1):84–105. https://doi.org/10.1016/j.pharmthera.2007.04.006.
Scott FL, et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173(11):1778–92. https://doi.org/10.1111/bph.13476.
Sandborn WJ, et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016;374(18):1754–62. https://doi.org/10.1056/NEJMoa1513248.
Sandborn WJ, et al. Long-term efficacy and safety of ozanimod in moderately to severely active ulcerative colitis: results from the open-label extension of the randomized; phase 2 TOUCHSTONE study. J Crohn’s Colitis. 2021. https://doi.org/10.1093/ecco-jcc/jjab012 (Epub ahead of print(13 January 2021)).
ClinicalTrials.gov. Open-label extension of RPC1063 as Therapy for moderate to severe ulcerative colitis. 2015. https://clinicaltrials.gov/ct2/show/NCT02531126. Accessed 8 Apr 2021.
Tran JQ, et al. Cardiac safety of ozanimod, a novel sphingosine-1-phosphate receptor modulator: results of a thorough QT/QTc study. Clin Pharmacol Drug Dev. 2018;7(3):263–76. https://doi.org/10.1002/cpdd.383.
ClinicalTrials.gov. Safety and efficacy trial of RPC1063 for moderate to severe ulcerative colitis. 2015. https://clinicaltrials.gov/ct2/show/NCT02435992. Accessed 8 Apr 2021.
Sandborn W, et al. P025 ozanimod efficacy, safety, and histology in patients with moderate-to-severe ulcerative colitis during induction in the phase 3 true north study. Am J Gastroenterol. 2020;115(Supplement_1):S6–7. https://doi.org/10.14309/01.ajg.0000722896.32651.d6.
Silvio D, et al. P030 ozanimod efficacy, safety, and histology in patients with moderate-to-severe ulcerative colitis during maintenance in the phase 3 true north study. Am J Gastroenterol. 2020;115:S8. https://doi.org/10.14309/01.ajg.0000722916.98351.89.
Feagan BG, et al. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: a single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol Hepatol. 2020;5(9):819–28. https://doi.org/10.1016/S2468-1253(20)30188-6.
Rutgeerts P, et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology. 2018;155(4):1045–58. https://doi.org/10.1053/j.gastro.2018.06.035.
Sandborn WJ, et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):550–61. https://doi.org/10.1053/j.gastro.2019.10.035.
Vermeire S, et al. Long-term safety and efficacy of etrasimod for ulcerative colitis: results from the open-label extension of the OASIS study. J Crohn’s Colitis. 2021. https://doi.org/10.1093/ecco-jcc/jjab016 (Epub ahead of print(22 January 2021)).
D’Haens G, et al. DOP48 Amiselimod, a selective S1P receptor modulator in Crohn’s disease patients: a proof-of-concept study. J Crohn’s Colitis. 2019;13(Supplement_1):S055–6. https://doi.org/10.1093/ecco-jcc/jjy222.082.
D’Haens GRAM, et al. P095 Amiselimod safety profile for Crohn’s disease, stratified by previous treatment with anti-TNF agents. Gastroenterology. 2020;158(Supplement_3):S1. https://doi.org/10.1053/j.gastro.2019.11.042.
D’Haens GRAM, et al. P097 Favorable safety profile for amiselimod, a selective S1P receptor modulator, in Crohn’s disease. Gastroenterology. 2020;158(Supplement_3):S2. https://doi.org/10.1053/j.gastro.2019.11.044.
Song J, et al. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharmacol Exp Ther. 2008;324(1):276–83. https://doi.org/10.1124/jpet.106.119172.
Radeke HH, et al. A multicentre, double-blind; placebo-controlled; parallel-group study to evaluate the efficacy, safety, and tolerability of the S1P receptor agonist KRP203 in patients with moderately active refractory ulcerative colitis. Inflamm Intest Dis. 2020;5(4):180–90. https://doi.org/10.1159/000509393.
Ma C, et al. Innovations in oral therapies for inflammatory bowel disease. Drugs. 2019;79(12):1321–35. https://doi.org/10.1007/s40265-019-01169-y.
D’Amico F, et al. New drugs in the pipeline for the treatment of inflammatory bowel diseases: what is coming? Curr Opin Pharmacol. 2020;55:141–50. https://doi.org/10.1016/j.coph.2020.10.015.
Zundler S, et al. The α4β1 homing pathway is essential for ileal homing of Crohn’s disease effector T cells in vivo. Inflamm Bowel Dis. 2017;23(3):379–91. https://doi.org/10.1097/mib.0000000000001029.
Yang E, et al. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn’s disease. Aliment Pharmacol Ther. 2020;51(11):1031–8. https://doi.org/10.1111/apt.15719.
Glassner K, et al. The use of combination biological or small molecule therapy in inflammatory bowel disease: a retrospective cohort study. J Dig Dis. 2020;21(5):264–71. https://doi.org/10.1111/1751-2980.12867.
Bristol Myers Squibb. Bristol Myers Squibb Presents Positive Late-Breaking Data from Phase 3 True North Trial Evaluating Zeposia (ozanimod) in Adult Patients with Moderate to Severe Ulcerative Colitis. Corporate/Financial News 2020. https://news.bms.com/news/details/2020/Bristol-Myers-Squibb-Presents-Positive-Late-Breaking-Data-from-Phase-3-True-North-Trial-Evaluating-Zeposia-ozanimod-in-Adult-Patients-with-Moderate-to-Severe-Ulcerative-Colitis/default.aspx. Accessed 23 Apr 2021
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflicts of interest
Dr R Alexander Speight has received grants from Genentech and personal fees from Dr Falk Pharma, Janssen, AbbVie, Tillotts and Takeda, outside the submitted work. Dr Nicola Wyatt, Dr Christopher Stewart, and Prof. John Kirby have no conflicts of interest that are directly relevant to the content of this article. Dr Christopher Lamb has received grants from Genentech, AbbVie, Eli Lilly, Pfizer, Roche, UCB Biopharma, Sanofi Aventis, Biogen IDEC, Orion OYJ and AstraZeneca; personal fees from Dr Falk Pharma and Ferring; and grants and personal fees from Janssen and Takeda, all outside the submitted work.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
NJW and CAL planned the manuscript content, which was drafted by NJW. CAL provided critical review during manuscript drafting and supervised the work. All other authors (RAS, CJS, and JAK) critically reviewed the manuscript. All authors approved the final manuscript.
Ethics approval
Not applicable.
Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Wyatt, N.J., Speight, R.A., Stewart, C.J. et al. Targeting Leukocyte Trafficking in Inflammatory Bowel Disease. BioDrugs 35, 473–503 (2021). https://doi.org/10.1007/s40259-021-00496-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-021-00496-5