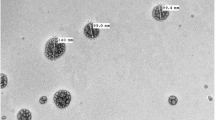
Abstract
Background and Objectives
Curcumin, an established pleiotropic agent, has potential for hepatoprotection owing to its powerful antioxidant, anti-inflammatory, and antifibrogenic properties. However, its poor bioavailability limits its use in therapeutics. In this study, we aimed to package curcumin into solid lipid nanoparticles (C-SLNs) to improve its bioavailability and compare the efficacy of C-SLNs with that of free curcumin and silymarin, a well-established hepatoprotectant in clinical use, against carbon tetrachloride (CCl4)-induced hepatic injury in rats, post-induction. A self-recovery group to which no treatment was given was also employed for quantifying self-healing of hepatic tissue, if any.
Material and Methods
C-SLNs (particle size 147.6 nm), prepared using a microemulsification technique, were administered to rats post-treatment with CCl4 (1 ml/kg body weight [BW] twice weekly for 2 weeks, followed by 1.5 ml/kg BW twice weekly for the subsequent 2 weeks). The extent of liver damage and repair in terms of histopathology and levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), oxidative stress markers (malondialdehyde, superoxide dismutase, and reduced glutathione) and a pro-inflammatory response marker, tumor necrosis factor (TNF)-α, were determined in both the CCl4 group and the treatment groups.
Results
C-SLNs (12.5 mg/kg) significantly (p < 0.001–0.005) attenuated histopathological changes and oxidative stress, and also decreased induction of ALT, AST, and TNF-α in comparison with free curcumin (100 mg/kg), silymarin (25 mg/kg), and self-recovery groups.
Conclusion
Curcumin could be used as a therapeutic agent for hepatic disorders, provided it is loaded into a suitable delivery system.






Similar content being viewed by others
References
Jayaprakasha G, Rao L, Sakariah K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006;98(4):720–4.
Tonnesen HH, Greenhil JVL. Studies on curcumin and curcuminoids XXII. Curcumin as a reducing agent and as a radical scavanger. Int J Pharm. 1992;87:79–87.
Reyes-Gordillo K, Segovia J, Shibayama M, Vergara P, Moreno MG, Muriel P. Curcumin protects against acute liver damage in the rat by inhibiting NF-kappaB, proinflammatory cytokines production and oxidative stress. Biochim Biophys Acta. 2007;1770(6):989–96.
Arora RB, Kapoor V, Basu N, Jain AP. Anti-inflammatory studies on Curcuma longa (turmeric). Indian J Med Res. 1971;59(8):1289–95.
Subramanian L, Selvam R, Mudaliar AL. Prevention of CCl4-induced hepatotoxicity by aqueous extract of tumeric. Nutr Res. 1999;19:429–41.
Tonnesen HH, Masson M, Loftsson T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharm. 2002;244(1–2):127–35.
Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15(12):1867–76.
Ammon HPT, Wahl MA. Pharmacology of Curcuma longa. Planta Medica. 1991;57:1–7.
Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7(7):1894–900.
Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330(1–2):155–63.
Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16(3):259–65.
Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–6.
Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104(6):1322–31.
Ma Z, Shayeganpour A, Brocks DR, Lavasanifar A, Samuel J. High-performance liquid chromatography analysis of curcumin in rat plasma: application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed Chromatogr. 2007;21(5):546–52.
Liu A, Lou H, Zhao L, Fan P. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. 2006;40(3):720–7.
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18.
Mathew A, Fukuda T, Nagaoka Y, Hasumura T, Morimoto H, Yoshida Y, Maekawa T, Venugopal K, Kumar DS. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE. 2012;7(3):e32616. doi:10.1371/journal.pone.0032616.
Yadav A, Lomash V, Samim M, Flora SJ. Curcumin encapsulated in chitosan nanoparticles: a novel strategy for the treatment of arsenic toxicity. Chem Biol Interact. 2012;199(1):49–61.
Grama CN, Suryanarayana P, Patil MA, Raghu G, Balakrishna N, et al. Efficacy of biodegradable curcumin nanoparticles in delaying cataract in diabetic rat model. PLoS ONE. 2013;8(10):e78217. doi:10.1371/journal.pone.0078217.
Yallapu MM, Othman SF, Curtis ET, Bauer NA, Chauhan N, Kumar D, Jaggi M, Chauhan SC. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomed. 2012;7:1761–79.
Yallapu MM, Ebeling MC, Khan S, Sundram V, Chauhan N, Gupta BK, Puumala SE, Jaggi M, Chauhan SC. Novel curcumin-loaded magnetic nanoparticles for pancreatic cancer treatment. Mol Cancer Ther. 2013;12(8):1471–80. doi:10.1158/1535-7163.
Sandhir R, Yadav A, Mehrotra A, Sunkaria A, Singh A, Sharma S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. Neuro Mol Med. 2013. doi:10.1007/s12017-013-8261-y.
Bisht S, Khan MA, Bekhit M, Bai H, Cornish T, Mizuma M, Rudek MA, Zhao M, Maitra A, Ray B, Lahiri D, Maitra A, Anders RA. A polymeric nanoparticle formulation of curcumin (NanoCurc™) ameliorates CCl4-induced hepatic injury and fibrosis through reduction of pro-inflammatory cytokines and stellate cell activation. Lab Invest. 2011;91(9):1383–95.
Sankar P, Gopal Telang A, Kalaivanan R, Karunakaran V, Manikam K, Sarkar SN. Effects of nanoparticle-encapsulated curcumin on arsenic-induced liver toxicity in rats. Environ Toxicol. 2013. doi:10.1002/tox.21940.
Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles for brain targeting. J Control Release. 2008;127:97–109.
Kakkar V, Singh S, Singla D, Kaur IP. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol Nutr Food Res. 2011;55(3):495–503.
Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs—a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113(1–3):151–70.
Jores K, Mehnert W, Drechsler M, Bunjes H, Johann C, Mader K. Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J Control Release. 2004;95(2):217–27.
Hu L, Tang X, Cui F. Solid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugs. J Pharm Pharmacol. 2004;56(12):1527–35.
Hu L, Xing Q, Meng J, Shang C. Preparation and enhanced oral bioavailability of cryptotanshinone-loaded solid lipid nanoparticles. AAPS PharmSciTech. 2010;11(2):582–7.
Freitas C, Muller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN (TM)) dispersions. Int J Pharm. 1998;168:221–9.
Rivera-Espinoza Y, Muriel P. Pharmacological actions of curcumin in liver diseases or damage. Liver Int. 2009;29(10):1457–66.
Muriel P, Rivera-Espinoza Y. Beneficial drugs for liver diseases. J Appl Toxicol. 2008;28(2):93–103.
Rechnagel RO, Glende EA Jr. Carbon tetrachloride hepatotoxicity: an example of lethal cleavage. CRC Crit Rev Toxicol. 1973;2(3):263–97.
Perez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983;3(1):112–20.
Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I, III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5(5):397–403.
Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124(5):491–504.
Kakkar V, Kaur IP. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem Toxicol. 2011;49(11):2906–13.
Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853(1–2):183–9.
Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh). 1978;43(2):86–92.
Manjunath K, Reddy JS, Venkateswarlu V. Solid lipid nanoparticles as drug delivery systems. Methods Find Exp Clin Pharmacol. 2005;27(2):127–44.
Gopal N, Sengottuvelu S. Hepatoprotective activity of Clerodendrum inerme against CCl4 induced hepatic injury in rats. Fitoterapia. 2008;79(1):24–6.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63.
Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99(3):667–76.
Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186(1):189–95.
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–69.
Morisco F, Vitaglione P, Amoruso D, Russo B, Fogliano V, Caporaso N. Foods and liver health. Mol Aspects Med. 2008;29(1–2):144–50.
Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222(1):1–15.
Liu SL, Degli Esposti S, Yao T, Diehl AM, Zern MA. Vitamin E therapy of acute CCl4-induced hepatic injury in mice is associated with inhibition of nuclear factor kappa B binding. Hepatology. 1995;22(5):1474–81.
Hernandez-Munoz R, Diaz-Munoz M, Chagoya de Sanchez V. Possible role of cell redox state on collagen metabolism in carbon tetrachloride-induced cirrhosis as evidenced by adenosine administration to rats. Biochim Biophys Acta. 1994;1200(2):93–9.
Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105–36.
Czaja MJ, Xu J, Alt E. Prevention of carbon tetrachloride-induced rat liver injury by soluble tumor necrosis factor receptor. Gastroenterology. 1995;108(6):1849–54.
Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73(2):399–409.
He SM, Chan E, Zhou SF. ADME properties of herbal medicines in humans: evidence, challenges and strategies. Curr Pharm Des. 2011;17(4):357–407.
Bhandari R, Kaur IP. Pharmacokinetics, tissue distribution and relative bioavailability of isoniazid-solid lipid nanoparticles. Int J Pharm. 2013;441:202–12.
Bhandari R, Kaur IP. A method to prepare solid lipid nanoparticles with improved entrapment efficiency of hydrophilic drugs. Curr Nanosci. 2013;9:211–20.
Hu Y, Xie J, Tong YW, Wang CH. Effect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cells. J Control Release. 2007;118(1):7–17.
Liang HF, Yang TF, Huang CT, Chen MC, Sung HW. Preparation of nanoparticles composed of poly(gamma-glutamic acid)-poly(lactide) block copolymers and evaluation of their uptake by HepG2 cells. J Control Release. 2005;105(3):213–25.
Hashida M, Takemura S, Nishikawa M, Takakura Y. Targeted delivery of plasmid DNA complexed with galactosylated poly(l-lysine). J Control Release. 1998;53(1–3):301–10.
Li L, Wang H, Ong ZY, Xu K, Ee PLR, Zheng S, Hedrick JL, Yang YY. Polymer- and lipid-based nanoparticle therapeutics for the treatment of liver diseases. Nano Today. 2010;5:296–312.
He J, Hou S, Lu W, Zhu L, Feng J. Preparation, pharmacokinetics and body distribution of silymarin-loaded solid lipid nanoparticles after oral administration. J Biomed Nanotechnol. 2007;3(4):195–202.
Kakkar V, Kaur IP. Antidepressant activity of curcumin loaded solid lipid nanoparticles (C-SLNs) in mice. Am J Pharm Tech Res. 2012;2(3):34–46.
Kakkar V, Muppu SK, Chopra K, Kaur IP. Curcumin loaded solid lipid nanoparticles: an efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur J Pharm Biopharm. 2013;6411(13):00059.
Kakkar V, Mishra AK, Chuttani K, Kaur IP. Proof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles (C-SLNs) to brain. Int J Pharm. 2013;448(2):354–9.
Dong MX, Jia Y, Zhang YB, Li CC, Geng YT, Zhou L, et al. Emodin protects rat liver from CCl(4)-induced fibrogenesis via inhibition of hepatic stellate cells activation. World J Gastroenterol. 2009;15(38):4753–62.
Sallie R, Tredger JM, Williams R. Drugs and the liver. Part 1: Testing liver function. Biopharm Drug Dispos. 1991;12(4):251–9.
Javed S, Kohli K, Ali M. Reassessing bioavailability of silymarin. Altern Med Rev. 2011;16(3):239–49.
Mansour MA. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci. 2000;66(26):2583–91.
Barry J, Fritz M, Brender JR, Smith PE, Lee DK, Ramamoorthy A. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: the case of the antioxidant curcumin. J Am Chem Soc. 2009;131(12):4490–8.
Curtis SJ, Moritz M, Snodgrass PJ. Serum enzymes derived from liver cell fractions. I. The response to carbon tetrachloride intoxication in rats. Gastroenterology. 1972;62(1):84–92.
Feher J, Lengyel G. Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Curr Pharm Biotechnol. 2012;13(1):210–7.
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–92.
Blair IA. Endogenous glutathione adducts. Curr Drug Metab. 2006;7(8):853–72.
Shukitt-Hale B, Erat SA, Joseph JA. Spatial learning and memory deficits induced by dopamine administration with decreased glutathione. Free Radic Biol Med. 1998;24(7–8):1149–58.
Roy S, Sannigrahi S, Majumdar S, Ghosh B, Sarkar B. Resveratrol regulates antioxidant status, inhibits cytokine expression and restricts apoptosis in carbon tetrachloride induced rat hepatic injury. Oxid Med Cell Longev. 2011;2011(703676):15.
Hernandez-Munoz I, de la Torre P, Sanchez-Alcazar JA, Garcia I, Santiago E, Munoz-Yague MT, et al. Tumor necrosis factor alpha inhibits collagen alpha 1(I) gene expression in rat hepatic stellate cells through a G protein. Gastroenterology. 1997;113(2):625–40.
Berghe TV, Denecker G, Brouckaert G, Vadimovisch Krysko D, D’Herde K, Vandenabeele P. More than one way to die: methods to determine TNF-induced apoptosis and necrosis. Methods Mol Med. 2004;98:101–26.
Acknowledgments
The Senior Research Fellowship and contingent grant provided to Neha Singh by the Indian Council of Medical Research (ICMR), New Delhi, India, is gratefully acknowledged. The authors are thankful to Mr. Dinesh Sharma and the Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh, India, for transmission electron microscopic analysis. The authors declare that they have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, N., Khullar, N., Kakkar, V. et al. Attenuation of Carbon Tetrachloride-Induced Hepatic Injury with Curcumin-Loaded Solid Lipid Nanoparticles. BioDrugs 28, 297–312 (2014). https://doi.org/10.1007/s40259-014-0086-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-014-0086-1