Abstract
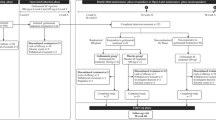
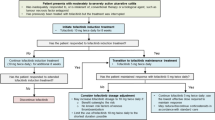
Adalimumab is a fully human, recombinant, monoclonal IgG1 antibody specific for the cytokine tumor necrosis factor-α. It is approved for the treatment of patients with inflammatory diseases, including adults with moderately to severely active ulcerative colitis who are refractory to, or intolerant of, corticosteroids and/or immunomodulators. In two well-designed 8- and 52-week clinical trials in patients with moderately to severely active ulcerative colitis despite treatment with corticosteroids and/or immunomodulators, subcutaneous adalimumab (160 mg, week 0; 80 mg, week 2; 40 mg every other week starting at week 4) was more effective than placebo for inducing and maintaining clinical remission. A statistically significant effect size (albeit <10 %) over placebo for the remission per Mayo score (primary endpoint) was observed with adalimumab at 8 weeks in both trials and at 52 weeks in one trial. Compared with placebo, adalimumab was associated with reductions in hospitalizations and improvements in other secondary endpoints, including clinical response, mucosal healing, corticosteroid-sparing, and health-related quality of life measures. Additionally, an early response to adalimumab was shown to be predictive of long-term efficacy. Adalimumab was generally well tolerated, compared with placebo, during clinical trials in patients with ulcerative colitis; the adverse event profile was similar to that in patients with Crohn’s disease or other approved indications. Adalimumab provides a new treatment option for patients with moderately to severely active ulcerative colitis who are refractory to, or intolerant of, corticosteroids and/or immunomodulators.

Similar content being viewed by others
References
Stange EF, Travis SP, Vermeire S, et al. European evidence-based consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis. 2008;2(1):1–23.
Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501–23.
Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365(18):1713–25.
Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–17.
Cohen RD, Yu AP, Wu EQ, et al. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31(7):693–707.
Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369(9573):1627–40.
Van Deventer SJ. Tumour necrosis factor and Crohn’s disease. Gut. 1997;40(4):443–8.
Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1756–67.
Sands BE, Kaplan GG. The role of TNFα in ulcerative colitis. J Clin Pharmacol. 2007;47(8):930–41.
Travis SP, Stange EF, Lemann M, et al. European evidence-based consensus on the management of ulcerative colitis: current management. J Crohns Colitis. 2008;2(1):24–62.
Janssen Biotech. Remicade (infliximab): US prescribing information. 2011. http://www.remicade.com/hcp/prescribing-information. Accessed 25 Mar 2013.
Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348(7):601–8.
Zabana Y, Domenech E, Manosa M, et al. Infliximab safety profile and long-term applicability in inflammatory bowel disease: 9-year experience in clinical practice. Aliment Pharmacol Ther. 2010;31(5):553–60.
European Medicines Agency. Humira 40 mg solution for injection: summary of product characteristics. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000481/WC500050870.pdf. Accessed 25 Mar 2013.
Abbott Laboratories. Humira (adalimumab): US prescribing information. 2012. http://www.rxabbott.com/pdf/humira.pdf. Accessed 25 Mar 2013.
Bang LM, Keating GM. Adalimumab: a review of its use in rheumatoid arthritis. BioDrugs. 2004;18(2):121–39.
Croom KF, McCormack PL. Adalimumab: in plaque psoriasis. Am J Clin Dermatol. 2009;10(1):43–50.
Cvetkovic RS, Scott LJ. Adalimumab: a review of its use in adult patients with rheumatoid arthritis. BioDrugs. 2006;20(5):293–311.
Plosker GL, Lyseng-Williamson KA. Adalimumab: in Crohn’s disease. BioDrugs. 2007;21(2):125–32.
Simpson D, Scott LJ. Adalimumab: in psoriatic arthritis. Drugs. 2006;66(11):1487–96.
Burness CB, Deeks ED. Adalimumab: in non-radiographic axial spondyloarthritis. Drugs. 2012;72(18):2385–95.
Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–7.
Reinisch W, Sandborn W, Hommes D, et al. Effect of adalimumab induction therapy on clinical laboratory parameters suggesting improved nutrition and inflammation status in patients with moderately to severely active ulcerative colitis [abstract no. P292 plus poster]. Inflamm Bowel Dis. 2011;17(Suppl 1):S58–9.
Werner L, Berndt U, Paclik D, et al. TNFα inhibitors restrict T cell activation and cycling via Notch-1 signalling in inflammatory bowel disease. Gut. 2012;61(7):1016–27.
Emi Aikawa N, de Carvalho JF, Artur Almeida Silva C, et al. Immunogenicity of anti-TNF-α agents in autoimmune diseases. Clin Rev Allergy Immunol. 2010;38(2–3):82–9.
van Schouwenburg PA, van de Stadt LA, de Jong RN, et al. Adalimumab elicits a restricted anti-idiotypic antibody response in autoimmune patients resulting in functional neutralisation. Ann Rheum Dis. 2013;72(1):104–9.
Eriksson C, Engstrand S, Sundqvist KG, et al. Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNF alpha. Ann Rheum Dis. 2005;64(3):403–7.
Beigel F, Schnitzler F, Paul Laubender R, et al. Formation of antinuclear and double-strand DNA antibodies and frequency of lupus-like syndrome in anti-TNF-alpha antibody-treated patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(1):91–8.
Awni W, Eckert D, Sharma S, et al. Pharmacokinetics of adalimumab in adult patients with moderately to severely active ulcerative colitis [abstract no. P412 plus poster]. In: 8th Congress of the European Crohn’s and Colitis Organisation (ECCO), Vienna, Austria; 14–16 Feb 2013.
Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257–65.e1–3.
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) assessment report for Humira (adalimumab): procedure no. EMEA/H/C/000481/II/0082. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000481/WC500126007.pdf. Accessed 25 Mar 2013.
Keizer RJ, Huitema AD, Schellens JH, et al. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493–507.
Colombel JF, Sandborn W, Wolf D, et al. Long-term efficacy of adalimumab for treatment of moderately to severely active ulcerative colitis [abstract no. OP349 plus oral presentation]. In: 20th United European Gastroenterology Week, Amsterdam, The Netherlands; 20–24 Oct 2012.
Reinisch W, Sandborn WJ, Kumar A, et al. 52-Week clinical efficacy with adalimumab in patients with moderately to severely active ulcerative colitis who failed corticosteroids and/or immunosuppressants [abstract no. PWE-032 plus poster]. Gut. 2011;60(Suppl 1):A139–40.
Sandborn WJ, Van Assche G, Thakkar RB, et al. Adalimumab improves health-related quality of life for 52 weeks in patients with ulcerative colitis [abstract no. P181 plus poster]. In: 6th Congress of the European Crohn’s and Colitis Organisation (ECCO), Dublin, Ireland; 24–26 Feb 2011.
Ghosh S, Wolf D, Sandborn W, et al. Sustained efficacy in patients with ulcerative colitis treated with adalimumab: results from ULTRA 2 [abstract no. P568 plus poster]. In: 8th Congress of the European Crohn’s and Colitis Organisation (ECCO), Vienna, Austria; 14–16 Feb 2013.
Lewis J, Ghosh S, Sandborn W, et al. Rates of “patient-defined” remission with adalimumab in patients with ulcerative colitis: subanalysis of ULTRA 1 and ULTRA 2 [abstract no. P319 plus poster]. In: 8th Congress of the European Crohn’s and Colitis Organisation (ECCO), Vienna, Austria; 14–16 Feb 2013.
Feagan BG, Sandborn WJ, Yang M, et al. Adalimumab therapy reduces hospitalization and colectomy rates in patients with ulcerative colitis: data from controlled trials [abstract no. OP209 plus poster]. In: 19th United European Gastroenterology Week, Stockholm, Sweden; 22–26 Oct 2011.
Feagan B, Sandborn W, Lazar A, et al. Adalimumab induction dose reduces the risk of hospitalizations and colectomies in patients with ulcerative colitis during the first 8 weeks of therapy [abstract no. 1601 plus poster]. Am J Gastroenterol. 2012;107(Suppl 1):S647.
Feagan B, Sandborn W, Yang M, et al. Adalimumab therapy reduces hospitalization and colectomy rates in patients with ulcerative colitis among initial responders [abstract no. 1592 plus poster]. Am J Gastroenterol. 2012;107(Supp 1):S642–3.
Sandborn W, Thakkar R, Lazar A, et al. Time-in-remission analysis of adalimumab vs. placebo for the treatment of ulcerative colitis [abstract no. 1590]. Am J Gastroenterol. 2012;107(Suppl 1):S641–2.
Wolf D, D’Haens G, Sandborn W, et al. Rate of and response to dose escalation in patients treated with adalimumab for moderately to severely active ulcerative colitis: subanalysis of ULTRA 2 [abstract no. 1541]. Am J Gastroenterol. 2012;107(Suppl 1):S619.
Sandborn W, Reinisch W, Thakkar R, et al. Adalimumab induction therapy improves health-related quality of life in patients with moderately to severely active ulcerative colitis [abstract no. P-159 plus poster]. Inflamm Bowel Dis. 2011;17(Suppl 1):S60–1.
Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14(12):1660–6.
Sandborn WJ, Colombel J-F, D’Haens G, et al. One-year maintenance outcomes among patients with moderately-to-severely active ulcerative colitis who responded to induction therapy with adalimumab: subgroup analyses from ULTRA 2. Aliment Pharmacol Ther. 2013;37(2):204–13.
Abbott. Long-term open-label safety and efficacy study of adalimumab in subjects with ulcerative colitis [ClinicalTrials.gov identifier NCT00573794] US National Institutes of Health, ClinicalTrials.gov. 2012. http://www.clinicaltrials.gov/ct2/show/NCT00573794. Accessed 25 Mar 2012.
Colombel JF, Ghosh S, Sandborn W, et al. Safety of adalimumab in global clinical trials of ulcerative colitis patients [abstract no. P378 plus poster]. In: 8th Congress of the European Crohn’s and Colitis Organisation (ECCO), Vienna, Austria; 14–16 Feb 2013.
Food and Drug Administration (FDA). FDA briefing information for the August 28, 2012 meeting of the Gastrointestinal Drugs Advisory Committee. 2012. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugsAdvisoryCommittee/UCM316786.pdf. Accessed 25 Mar 2013.
Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2012;. doi:10.1136/annrheumdis-2011-201244.
Deepak P, Sifuentes H, Sherid M, et al. T-cell non-Hodgkin’s lymphomas reported to the FDA AERS with tumor necrosis factor-alpha (TNF-alpha) inhibitors: results of the REFURBISH study. Am J Gastroenterol. 2012;. doi:10.1038/ajg.2012.334.
Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146(12):829–38.
Ali T, Skup M, Yang M, et al. Cost-effectiveness of adalimumab in moderately to severely active ulcerative colitis [abstract no. 1588]. Am J Gastroenterol. 2012;107(Suppl 1):S641.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: V. Annese, Unit of Gastroenterology SOD2, Azienda Ospedaliero Universitaria (AOU) Careggi, University Hospital, Florence, Italy; D.B. Sachar, Division of Gastroenterology, Mount Sinai School of Medicine, New York, New York, USA; B. Shen, Department of Gastroenterology and Hepatology, Cleveland Clinic Foundation, Cleveland, Ohio, USA.
Rights and permissions
About this article
Cite this article
Burness, C.B., Keating, G.M. Adalimumab: A Review of Its Use in the Treatment of Patients with Ulcerative Colitis. BioDrugs 27, 247–262 (2013). https://doi.org/10.1007/s40259-013-0033-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-013-0033-6