Abstract
Background
Numerous diagnostic criteria for atopic dermatitis are used in clinical trials, which may limit comparison of results.
Objective
We sought to determine the most commonly used atopic dermatitis diagnostic criteria in randomized controlled trials internationally.
Methods
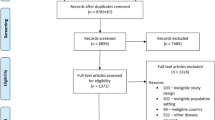
We performed a systematic review of randomized controlled trials with a pharmacological intervention from 2007 to 2016. Cochrane Library, EMBASE, GREAT, LILACS, MEDLINE, and Scopus were searched. Two authors independently performed the study selection and data extraction.
Results
Two hundred and twelve randomized controlled trials met inclusion/exclusion criteria. Overall, ten different diagnostic criteria were used. The Hanifin and Rajka criteria were most commonly used (41.0%), followed by the UK refinement of the Hanifin and Rajka criteria (9.0%), Japanese Dermatological Association criteria (4.2%), and American Academy of Dermatology criteria (3.8%). No diagnostic criteria were specified in 37.3% of randomized controlled trials. The Hanifin and Rajka criteria were the most commonly used atopic dermatitis diagnostic criteria in clinical trials of topical and systemic interventions, across all years between 2007 and 2016, in pediatric and adult populations, in most countries and regions internationally.
Conclusions
The results highlight the lack of uniformity and documentation of atopic dermatitis diagnostic criteria in randomized controlled trials for atopic dermatitis. We recommend harmonizing the diagnostic criteria for atopic dermatitis in future randomized controlled trials.



Similar content being viewed by others
References
Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI, Group IPTS. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;24(6):1251.e23–1258.e23.
Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33(3):281–8.
Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Investig Dermatol. 2015;135(1):56–66.
Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132(5):1132–8.
Bieber T. Atopic dermatitis 2.0: from the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy. 2012;67(12):1475–82.
Silverberg JI. Racial and ethnic disparities in atopic dermatitis. Curr Dermatol Rep. 2015;4(1):44–8.
Noda S, Suarez-Farinas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136(5):1254–64.
Hanifin J, Rajka G. Diagnostic features of atopic eczema. Acta Dermato-Venereol. 1980;92:44–7.
Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4(5):884–917.
Andersen RM, Thyssen JP, Maibach HI. Qualitative vs. quantitative atopic dermatitis criteria: in historical and present perspectives. J Eur Acad Dermatol Venereol. 2016;30(4):604–18.
Brenninkmeijer EE, Schram ME, Leeflang MM, Bos JD, Spuls PI. Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol. 2008;158(4):754–65.
De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20(7):853–9.
Johnke H, Vach W, Norberg LA, Bindslev-Jensen C, Host A, Andersen KE. A comparison between criteria for diagnosing atopic eczema in infants. Br J Dermatol. 2005;153(2):352–8.
Roguedas AM, Machet L, Fontes V, Lorette G. Atopic dermatitis: which are the diagnostic criteria used in medical literature? Ann Dermatol Venereol. 2004;131(2):161–4.
Bos JD, Van Leent EJ, Sillevis Smitt JH. The millennium criteria for the diagnosis of atopic dermatitis. Exp Dermatol. 1998;7(4):132–8.
Diepgen TL, Sauerbrei W, Fartasch M. Development and validation of diagnostic scores for atopic dermatitis incorporating criteria of data quality and practical usefulness. J Clin Epidemiol. 1996;49(9):1031–8.
Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–91.
Williams HC, Burney PG, Hay RJ, et al. The U.K. Working Party’s diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol. 1994;131(3):383–96.
Schultz Larsen F, Hanifin JM. Secular change in the occurrence of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1992;176:7–12.
Kang KF, Tian RM. Criteria for atopic dermatitis in a Chinese population. Acta Derm Venereol Suppl (Stockh). 1989;144:26–7.
Tagami H. Japanese Dermatological Association criteria for the diagnosis of atopic dermatitis. J Dermatol. 2002;29(6):398.
Schultz Larsen F, Diepgen T, Svensson A. Clinical criteria in diagnosing atopic dermatitis: the Lillehammer criteria 1994. Acta Derm Venereol Suppl (Stockh). 1996;96:115–9.
Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113(5):832–6.
Nankervis H, Pynn EV, Boyle RJ, et al. House dust mite reduction and avoidance measures for treating eczema. Cochrane Database Syst Rev. 2015;1:CD008426.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Gu H, Chen XS, Chen K, et al. Evaluation of diagnostic criteria for atopic dermatitis: validity of the criteria of Williams et al in a hospital-based setting. Br J Dermatol. 2001;145(3):428–33.
Hanifin JM, Cooper KD, Ho VC, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association “Administrative Regulations for Evidence-Based Clinical Practice Guidelines”. J Am Acad Dermatol. 2004;50(3):391–404.
Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Investig Dermatol. 2015;135(1):24–30.
Schmitt J, Williams H. Harmonising Outcome Measures for Eczema (HOME). Report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatol. 2010;163(6):1166–8.
Flohr C. Atopic dermatitis diagnostic criteria and outcome measures for clinical trials: still a mess. J Investig Dermatol. 2011;131(3):557–9.
Bhattacharya T, Silverberg JI. Efficacy of systemic treatments for atopic dermatitis in racial and ethnic minorities in the United States. JAMA Dermatol. 2014;150(11):1232–4.
Henderson MD, Abboud J, Cogan CM, et al. Skin-of-color epidemiology: a report of the most common skin conditions by race. Pediatr Dermatol. 2012;29(5):584–9.
Bai Y, Yang D, Wang Y. Clinical study on treatment of acute eczema by Shuangfujin. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(1):72–5.
Wu Q, Chen L. Clinical observation of adult chronic eczema treated with clearing heat 25 Wei Wan combined triamcinolone acetonide. Clin J Med Off. 2008;36:267–8.
Park Y, Kim H, Kim K, et al. Report from ADRG: a study on the diagnostic criteria of Korean atopic dermatitis. Korean J Dermatol. 2006;44(6):659–63.
Seymour JL, Keswick BH, Hanifin JM, Jordan WP, Milligan MC. Clinical effects of diaper types on the skin of normal infants and infants with atopic dermatitis. J Am Acad Dermatol. 1987;17(6):988–97.
Acknowledgements
The authors thank Yufan Yan, BS, Northwestern University Feinberg School of Medicine for providing assistance with translation services.
Author information
Authors and Affiliations
Contributions
JIS had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: JIS. Acquisition of data: PPV, RC. Analysis and interpretation of data: JIS, PPV, RC. Drafting of the manuscript: JIS, PPV, RC. Critical revision of the manuscript for important intellectual content: JIS, PPV, RC. Statistical analysis: PPV, JIS. Obtained funding: JIS.
Corresponding author
Ethics declarations
Funding
This publication was made possible with support from the Agency for Healthcare Research and Quality, Grant No. K12 HS023011, and the Dermatology Foundation.
Conflict of interest
Jonathan I. Silverberg, Paras P. Vakharia, and Rishi Chopra have no conflicts of interest directly relevant to the contents of this study.
Ethics approval
This study was exempt from institutional review board approval.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vakharia, P.P., Chopra, R. & Silverberg, J.I. Systematic Review of Diagnostic Criteria Used in Atopic Dermatitis Randomized Controlled Trials. Am J Clin Dermatol 19, 15–22 (2018). https://doi.org/10.1007/s40257-017-0299-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-017-0299-4