Abstract
Background
Pemafibrate is a novel fibrate class drug that is a highly potent and selective agonist of peroxisome proliferator-activated receptor α (PPARα). We performed the first ever network meta-analysis containing the largest ever group of patients to test the efficacy of pemafibrate in improving lipid levels compared with fenofibrate and placebo in patients with dyslipidemia.
Methods
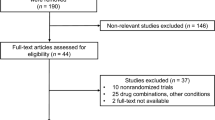
Potentially relevant clinical trials were identified in Medline, PubMed, Embase, clinicaltrials.gov, and Cochrane Controlled Trials registry. Nine randomized controlled trials met the inclusion criteria out of 40 potentially available articles. The primary effect outcome was a change in the levels of triglycerides (TG), high-density lipoproteins (HDL), or low-density lipoproteins (LDL) before and after the treatment.
Results
A total of 12,359 subjects were included. The mean patient age was 54.73 (years), the mean ratio for female patients was 18.75%, and the mean examination period was 14.22 weeks. The dose for pemafibrate included in our study was 0.1, 0.2, or 0.4 mg twice daily, whereas the dose for fenofibrate was 100 mg/day. Data showed a significant reduction in TG and a mild increase in HDL levels across the pemafibrate group at different doses and fenofibrate 100 mg group (with greatest effect observed with pemafibrate 0.1 mg twice daily). A mild increase in LDL was also observed in all groups, but the increase in LDL in the 0.1 mg twice daily dose group was statistically insignificant.
Conclusion
Pemafibrate 0.1 mg twice daily dose led to highest reduction in TG levels and the highest increase in HDL levels compared with other doses of pemafibrate, fenofibrate, and placebo.








Similar content being viewed by others
References
Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med. 2017;167(11):ITC81–96. https://doi.org/10.7326/AITC201712050.
Miller M. Dyslipidemia and cardiovascular risk: the importance of early prevention. QJM. 2009;102(9):657–67. https://doi.org/10.1093/qjmed/hcp065.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168–209. https://doi.org/10.1016/j.jacc.2018.11.002.
Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 1986;256(20):2823–8.
Kuklina EV, Yoon PW, Keenan NL. Trends in high levels of low-density lipoprotein cholesterol in the United States, 1999–2006. JAMA. 2009;302(19):2104–10. https://doi.org/10.1001/jama.2009.1672.
Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78. https://doi.org/10.1016/S0140-6736(05)67394-1.
Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81. https://doi.org/10.1016/S0140-6736(10)61350-5.
Mora S, Wenger NK, Demicco DA, et al. Determinants of residual risk in secondary prevention patients treated with high- versus low-dose statin therapy: the Treating to New Targets (TNT) study. Circulation. 2012;125(16):1979–87. https://doi.org/10.1161/CIRCULATIONAHA.111.088591.
Lim S, Park YM, Sakuma I, Koh KK. How to control residual cardiovascular risk despite statin treatment: focusing on HDL-cholesterol. Int J Cardiol. 2013;166(1):8–14. https://doi.org/10.1016/j.ijcard.2012.03.127.
Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–8. https://doi.org/10.1161/CIRCULATIONAHA.106.637793.
Abourbih S, Filion KB, Joseph L, et al. Effect of fibrates on lipid profiles and cardiovascular outcomes: a systematic review. Am J Med. 2009;122(10):962.e1-962.e9628. https://doi.org/10.1016/j.amjmed.2009.03.030.
Yamazaki Y, Abe K, Toma T, et al. Design and synthesis of highly potent and selective human peroxisome proliferator-activated receptor alpha agonists. Bioorg Med Chem Lett. 2007;17(16):4689–93. https://doi.org/10.1016/j.bmcl.2007.05.066.
Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–41. https://doi.org/10.1016/s0895-4356(98)00131-0.
Altman DG, Bland JM. Detecting skewness from summary information. BMJ. 1996;313(7066):1200. https://doi.org/10.1136/bmj.313.7066.1200.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. http://www.cochrane-handbook.org. Accessed 1 Nov 2018
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Ishibashi S, Yamashita S, Arai H, et al. Effects of K-877, a novel selective PPARα modulator (SPPARMα), in dyslipidaemic patients: a randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis. 2016;249:36–43. https://doi.org/10.1016/j.atherosclerosis.2016.02.029.
Arai H, Yamashita S, Yokote K, et al. Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: a multicenter, placebo-controlled, double-blind, randomized trial. J Atheroscler Thromb. 2018;25(6):521–38. https://doi.org/10.5551/jat.44412.
Araki E, Yamashita S, Arai H, et al. Effects of pemafibrate, a novel selective pparα modulator, on lipid and glucose metabolism in patients with type 2 diabetes and hypertriglyceridemia: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2018;41(3):538–46. https://doi.org/10.2337/dc17-1589.
Arai H, Yamashita S, Yokote K, et al. Efficacy and safety of K-877, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), in combination with statin treatment: two randomised, double-blind, placebo-controlled clinical trials in patients with dyslipidaemia. Atherosclerosis. 2017;261:144–52. https://doi.org/10.1016/j.atherosclerosis.2017.03.032.
Ishibashi S, Arai H, Yokote K, et al. Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor α modulator, in patients with dyslipidemia: results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J Clin Lipidol. 2018;12(1):173–84. https://doi.org/10.1016/j.jacl.2017.10.006.
Matsuba I, Matsuba R, Ishibashi S, et al. Effects of a novel selective peroxisome proliferator-activated receptor-α modulator, pemafibrate, on hepatic and peripheral glucose uptake in patients with hypertriglyceridemia and insulin resistance. J Diabetes Investig. 2018;9(6):1323–32. https://doi.org/10.1111/jdi.12845.
Yamashita S, Arai H, Yokote K, et al. Effects of pemafibrate (K-877) on cholesterol efflux capacity and postprandial hyperlipidemia in patients with atherogenic dyslipidemia. J Clin Lipidol. 2018;12(5):1267-1279.e4. https://doi.org/10.1016/j.jacl.2018.06.010.
Ginsberg HN, Hounslow NJ, Senko Y, et al. Efficacy and safety of K-877 (pemafibrate), a selective PPARα modulator, in European patients on statin therapy. Diabetes Care. 2022;45(4):898–908. https://doi.org/10.2337/dc21-1288.
Das Pradhan A, Glynn RJ, Fruchart JC, et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med. 2022;387(21):1923–34. https://doi.org/10.1056/NEJMoa2210645.
Ida S, Kaneko R, Murata K. Efficacy and safety of pemafibrate administration in patients with dyslipidemia: a systematic review and meta-analysis. Cardiovasc Diabetol. 2019;18(1):38. https://doi.org/10.1186/s12933-019-0845-x.
Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996;37(5):907–25.
Auboeuf D, Rieusset J, Fajas L, et al. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46(8):1319–27. https://doi.org/10.2337/diab.46.8.1319.
Yamashita S, Masuda D, Matsuzawa Y. Pemafibrate, a new selective PPARα modulator: drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr Atheroscler Rep. 2020;22(1):5. https://doi.org/10.1007/s11883-020-0823-5.
Zhang Z, Diao P, Zhang X, Nakajima T, Kimura T, Tanaka N. Clinically relevant dose of pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), lowers serum triglyceride levels by targeting hepatic PPARα in mice. Biomedicines. 2022;10(7):1667. https://doi.org/10.3390/biomedicines10071667.
Tanaka N, Honda A. Pemafibrate for primary biliary cholangitis with dyslipidemia: A proposal of a new treatment from Japan. Hepatol Res. 2022;52(6):495–6. https://doi.org/10.1111/hepr.13770.
Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol. 2007;99(6A):3C-18C. https://doi.org/10.1016/j.amjcard.2006.11.016.
Gariot P, Barrat E, Mejean L, Pointel JP, Drouin P, Debry G. Fenofibrate and human liver. Lack of proliferation of peroxisomes. Arch Toxicol. 1983;53(2):151–63. https://doi.org/10.1007/BF00302723.
Hounslow N, Mair S, Suganami H, Nakamura M. Pemafibrate has high bioavailability and is principally excreted via the liver. Atheroscler Suppl. 2018;32:157. https://doi.org/10.1016/j.atherosclerosissup.2018.04.475.
Yokote K, Yamashita S, Arai H, et al. Long-term efficacy and safety of pemafibrate, a novel selective peroxisome proliferator-activated receptor-α modulator (SPPARMα), in dyslipidemic patients with renal impairment. Int J Mol Sci. 2019;20(3):706. https://doi.org/10.3390/ijms20030706.
Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088–93. https://doi.org/10.1161/01.cir.98.19.2088.
Fruchart JC. Pemafibrate (K-877), a novel selective peroxisome proliferator-activated receptor alpha modulator for management of atherogenic dyslipidaemia. Cardiovasc Diabetol. 2017;16(1):124. https://doi.org/10.1186/s12933-017-0602-y.
Staels B, Vu-Dac N, Kosykh VA, et al. Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J Clin Invest. 1995;95(2):705–12. https://doi.org/10.1172/JCI117717.
Wang CS, McConathy WJ, Kloer HU, Alaupovic P. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J Clin Invest. 1985;75(2):384–90. https://doi.org/10.1172/JCI111711.
Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J Biol Chem. 1997;272(45):28210–7. https://doi.org/10.1074/jbc.272.45.28210.
Yoshida M, Nakamura K, Miyoshi T, et al. Combination therapy with pemafibrate (K-877) and pitavastatin improves vascular endothelial dysfunction in dahl/salt-sensitive rats fed a high-salt and high-fat diet. Cardiovasc Diabetol. 2020;19(1):149. https://doi.org/10.1186/s12933-020-01132-2.
Vu-Dac N, Schoonjans K, Kosykh V, et al. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J Clin Invest. 1995;96(2):741–50. https://doi.org/10.1172/JCI118118.
Vu-Dac N, Schoonjans K, Laine B, Fruchart JC, Auwerx J, Staels B. Negative regulation of the human apolipoprotein A-I promoter by fibrates can be attenuated by the interaction of the peroxisome proliferator-activated receptor with its response element. J Biol Chem. 1994;269(49):31012–8.
Takei K, Nakagawa Y, Wang Y, et al. Effects of K-877, a novel selective PPARα modulator, on small intestine contribute to the amelioration of hyperlipidemia in low-density lipoprotein receptor knockout mice. J Pharmacol Sci. 2017;133(4):214–22. https://doi.org/10.1016/j.jphs.2017.02.003.
Hoogeveen RC, Gaubatz JW, Sun W, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34(5):1069–77. https://doi.org/10.1161/ATVBAHA.114.303284.
Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):3024-3025] [published correction appears in J Am Coll Cardiol. 2015 Dec 22;66(24):2812]. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934. https://doi.org/10.1016/j.jacc.2013.11.002
Lupien PJ, Brun D, Gagné C, Moorjani S, Bielman P, Julien P. Gemfibrozil therapy in primary type II hyperlipoproteinemia: effects on lipids, lipoproteins and apolipoproteins. Can J Cardiol. 1991;7(1):27–33.
Goldberg R, La Belle P, Zupkis R, Ronca P. Comparison of the effects of lovastatin and gemfibrozil on lipids and glucose control in non-insulin-dependent diabetes mellitus. Am J Cardiol. 1990;66(8):16B-21B. https://doi.org/10.1016/0002-9149(90)90436-5.
Pasternak RC, Brown LE, Stone PH, Silverman DI, Gibson CM, Sacks FM. Effect of combination therapy with lipid-reducing drugs in patients with coronary heart disease and “normal” cholesterol levels. A randomized, placebo-controlled trial. Harvard Atherosclerosis Reversibility Project (HARP) Study Group. Ann Intern Med. 1996;125(7):529–40. https://doi.org/10.7326/0003-4819-125-7-199610010-00001.
Tilly-Kiesi M, Tikkanen MJ. Low density lipoprotein density and composition in hypercholesterolaemic men treated with HMG CoA reductase inhibitors and gemfibrozil. J Intern Med. 1991;229(5):427–34. https://doi.org/10.1111/j.1365-2796.1991.tb00370.x.
de Graaf J, Hendriks JC, Demacker PN, Stalenhoef AF. Identification of multiple dense LDL subfractions with enhanced susceptibility to in vitro oxidation among hypertriglyceridemic subjects. Normalization after clofibrate treatment. Arterioscler Thromb. 1993;13(5):712–9. https://doi.org/10.1161/01.atv.13.5.712.
Ginsberg HN, Packard CJ, Chapman MJ, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42(47):4791–806. https://doi.org/10.1093/eurheartj/ehab551.
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
There are no financial relationships to disclose.
Funding
No external funding was used in the preparation of this manuscript.
Conflict of Interest
Muhammad Shayan Khan, Ghulam Mujtaba Ghumman, Abdul Baqi, Jay Shah, Muhammad Aziz, Tanveer Mir, Ayesha Tahir, Srinivas Katragadda, Hemindermeet Singh, Mohammed Taleb, and Syed Sohail Ali declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
As it was a meta-analysis, the study did not require approval by institutional review board.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Authors’ Contributions
Muhammad Shayan Khan: Conceptualization, software, formal analysis. Ghulam Mujtaba Ghumman: Data curation, writing—original draft. Abdul Baqi: Data curation. Jay Shah: Writing—reviewing and editing. Muhammad Aziz: Methodology, visualization. Tanveer Mir: Software, validation. Ayesha Tahir: Writing—reviewing and editing. Srinivas Katragadda: Resources, project administration. Hemindermeet Singh: Methodology, investigation. Mohammed Taleb and Syed Sohail Ali: Supervision.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, M.S., Ghumman, G.M., Baqi, A. et al. Efficacy of Pemafibrate Versus Fenofibrate Administration on Serum Lipid Levels in Patients with Dyslipidemia: Network Meta-Analysis and Systematic Review. Am J Cardiovasc Drugs 23, 547–558 (2023). https://doi.org/10.1007/s40256-023-00593-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00593-6