Abstract
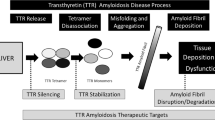
Transthyretin amyloid cardiomyopathy (ATTR-CM) is a progressive, life-threatening disease characterized by the aggregation and deposition of amyloidogenic misfolded transthyretin (TTR) in the myocardium. The gradual accumulation of insoluble TTR amyloid fibrils can result in restrictive cardiomyopathy and heart failure. Tafamidis (Vyndaqel®; Vyndamax®), a TTR stabilizer, has been approved for use in the treatment of adults with ATTR-CM in several countries. Tafamidis stabilizes both wild-type and mutant TTR, inhibiting the formation of TTR amyloid fibrils. In the pivotal phase III ATTR-ACT trial, tafamidis significantly reduced all-cause mortality and frequency of cardiovascular-related hospitalizations relative to placebo in patients with ATTR-CM. In addition, tafamidis recipients experienced significantly less deterioration in 6-minute walk test distance and quality of life than placebo recipients over the 30-month treatment period. Treatment benefits were largely consistent between patients with wild-type TTR and patients with a variant TTR genotype. Tafamidis was generally well tolerated in patients with ATTR-CM and, with a safety profile similar to that of placebo, tafamidis is suitable for long-term use. Given that treatment for this condition has in the past been largely limited to symptom management, tafamidis constitutes a valuable disease-modifying therapy for patients with ATTR-CM.

Similar content being viewed by others
References
Hafeez AS, Bavry AA. Diagnosis of transthyretin amyloid cardiomyopathy. Cardiol Ther. 2020;9:85–95.
Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709–16.
Castaño A, Drachman BM, Judge D, et al. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163–78.
Emdin M, Aimo A, Rapezzi C, et al. Treatment of cardiac transthyretin amyloidosis: an update. Eur Heart J. 2019;40(45):3699–706.
Bulawa CE, Connelly S, Devit M, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci USA. 2012;109(24):9629–34.
Pfizer. Vyndaqel: EU summary of product characteristics. 2020. https://www.ema.europa.eu/. Accessed 25 Nov 2020.
Pfizer. VYNDAQEL and VYNDAMAX: US prescribing information. 2020. http://www.pfizermedicalinformation.com/. Accessed 25 Nov 2020.
Pfizer. Japanese prescribing information (Vyndaqel®). 2019. https://www.pmda.go.jp/. Accessed 25 Nov 2020.
European Medicines Agency. Vyndaqel (tafamidis): CHMP assessment report. 2019. http://www.ema.europa.eu. Accessed 25 Nov 2020.
Lamb YN, Deeks ED. Tafamidis: a review in transthyretin amyloidosis with polyneuropathy. Drugs. 2019;79(8):863–74.
Scott LJ. Tafamidis: a review of its use in familial amyloid polyneuropathy. Drugs. 2014;74(12):1371–8.
Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–16.
Maurer MS, Grogan DR, Judge DP, et al. Tafamidis in transthyretin amyloid cardiomyopathy: effects on transthyretin stabilization and clinical outcomes. Circ Heart Fail. 2015;8(3):519–26.
Sultan MB, Gundapaneni B, Schumacher J, et al. Treatment with tafamidis slows disease progression in early-stage transthyretin cardiomyopathy. Clin Med Insights Cardiol. 2017;11:1–4.
Maurer MS, Elliott P, Merlini G, et al. Design and rationale of the phase 3 ATTR-ACT clinical trial (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial). Circ Heart Fail. 2017;10(6):1–7.
Damy T, Garcia-Pavia P, Hanna M, et al. Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study. Eur J Heart Fail. 2020. https://doi.org/10.1002/ejhf.2027.
Elliott P, Drachman BM, Gottlieb SS, et al. Interim analysis of data from a long-term, extension trial of tafamidis meglumine in patients with transthyretin amyloid cardiomyopathy [abstract no. 1169]. Eur Heart J. 2019;40(Suppl 1):645.
Miller AB, Januzzi J, O’Neill BJ, et al. Causes of cardiovascular hospitalization and death in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) [abstract no. 1089-09]. J Am Coll Cardiol. 2020;75(11 Suppl 1):692.
Hanna M, Damy T, Garcia-Pavia P, et al. Efficacy and safety of tafamidis doses in the tafamidis in transthyretin cardiomyopathy clinical trial (ATTR-ACT) [abstract no. 205]. J Card Fail. 2019;25(8 Suppl):S77–S8.
Li B, Alvir J, Stewart M. Extrapolation of survival benefits in patients with transthyretin amyloid cardiomyopathy receiving tafamidis: analysis of the tafamidis in transthyretin cardiomyopathy clinical trial. Cardiol Ther. 2020;9:535–40.
Hanna M, Stewart M, Gundapaneni B, et al. Tafamidis reduced the decline in health-related quality of life in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) [abstract no. 016]. J Card Fail. 2019;25(8 Suppl):S7.
Grogan M, Witteles R, Shah SJ, et al. Efficacy of tafamidis in patients with hereditary or wild-type transthyretin amyloid cardiomyopathy: further results from the ATTR-ACT trial [abstract no. 483]. J Heart Lung Transplant. 2019;38(4 Suppl):S204.
Shah SJ, Gundapaneni B, Sultan MB, et al. Survival benefit with higher-dose tafamidis in patients with hereditary and wild-type transthyretin amyloid cardiomyopathy [abstract no. 13149 and poster no. P629]. In: American Heart Association (AHA) Scientific Sessions 2020 (Nov 13–17). 2020.
Nativi-Nicolau J, van der Meer P, Gundapaneni B, et al. Tafamidis free acid 61 mg in patients with transthyretin amyloid cardiomyopathy [abstract no. 15528 and poster no. P630]. In: American Heart Association (AHA) Scientific Sessions 2020 (Nov 13–17). 2020.
Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(10):1169–86.
Rettl R, Duca F, Binder C, et al. Effects of tafamidis in patients with transthyretin amyloid cardiomyopathy [abstract no. P896]. Eur Heart J. 2019;40(Suppl 1):494.
Lockwood PA, Le VH, O’Gorman MT, et al. The bioequivalence of tafamidis 61-mg free acid capsules and tafamidis meglumine 4 × 20-mg capsules in healthy volunteers. Clin Pharmacol Drug Dev. 2020;9(7):849–54.
Kazi DS, Bellows BK, Baron SJ, et al. Cost-effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation. 2020;141(15):1214–24.
Rozenbaum MH, Kemner J, Parasuraman B. Letter by Rozenbaum et al. regarding article “Cost-effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy”. Circulation. 2020;142:e210–11.
Coelho T, Merlini G, Bulawa CE, et al. Mechanism of action and clinical application of tafamidis in hereditary transthyretin amyloidosis. Neurol Ther. 2016;5(1):1–25.
Klamerus KJ, Watsky E, Moller R, et al. The effect of tafamidis on the QTc interval in healthy subjects. Br J Clin Pharmacol. 2015;79(6):918–25.
Acknowledgements
During the peer review process, the manufacturer of tafamidis was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
Yvette Lamb is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: A. Aimo, University Hospital of Pisa, Pisa, Italy; E. Amiya, Department of Cardiovascular Medicine, Graduate School of Medicine, University of Tokyo, Tokyo, Japan.
Rights and permissions
About this article
Cite this article
Lamb, Y.N. Tafamidis: A Review in Transthyretin Amyloid Cardiomyopathy. Am J Cardiovasc Drugs 21, 113–121 (2021). https://doi.org/10.1007/s40256-020-00461-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-020-00461-7