Abstract
Background
AWAKE-HF evaluated the effect of the initiation of sacubitril/valsartan versus enalapril on activity and sleep using actigraphy in patients who have heart failure with reduced ejection fraction (HFrEF).
Methods
In this randomized, double-blind study, patients with HFrEF (n = 140) were randomly assigned to sacubitril/valsartan or enalapril for 8 weeks, followed by an 8-week open-label phase with sacubitril/valsartan. Primary endpoint was change from baseline in mean activity counts during the most active 30 min/day at week 8. The key secondary endpoint was change in mean nightly activity counts/minute from baseline to week 8. Kansas City Cardiomyopathy Questionnaire-23 (KCCQ-23) was an exploratory endpoint.
Results
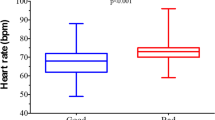
There were no detectable differences between groups in geometric mean ratio of activity counts during the most active 30 min/day at week 8 compared with baseline (0.9456 [sacubitril/valsartan:enalapril]; 95% confidence interval [CI] 0.8863–1.0088; P = 0.0895) or in mean change from baseline in activity during sleep (difference: 2.038 counts/min; 95% CI − 0.062 to 4.138; P = 0.0570). Change from baseline to week 8 in KCCQ-23 was 2.89 for sacubitril/valsartan and 4.19 for enalapril, both nonsignificant.
Conclusions
In AWAKE-HF, no detectable differences in activity and sleep were observed when comparing sacubitril/valsartan with enalapril in patients with HFrEF using a wearable biosensor.
Clinical trial registration
ClinicalTrials.gov, NCT02970669.





Similar content being viewed by others
References
Heo S, Doering LV, Widener J, Moser DK. Predictors and effect of physical symptom status on health-related quality of life in patients with heart failure. Am J Crit Care. 2008;17:124–32.
Barnes S, Gott M, Payne S, Parker C, Seamark D, Gariballa S, Small N. Prevalence of symptoms in a community-based sample of heart failure patients. J Pain Symptom Manage. 2006;32:208–16.
Howell J, Strong BM, Weisenberg J, Kakade A, Gao Q, Cuddihy P, Delisle S, Kachnowski S, Maurer MS. Maximum daily 6 minutes of activity: an index of functional capacity derived from actigraphy and its application to older adults with heart failure. J Am Geriatr Soc. 2010;58:931–6.
Conraads VM, Spruit MA, Braunschweig F, Cowie MR, Tavazzi L, Borggrefe M, Hill MR, Jacobs S, Gerritse B, van Veldhuisen DJ. Physical activity measured with implanted devices predicts patient outcome in chronic heart failure. Circ Heart Fail. 2014;7:279–87.
Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012;126:1495–510.
Melin M, Hagerman I, Gonon A, Gustafsson T, Rullman E. Variability in physical activity assessed with accelerometer is an independent predictor of mortality in CHF patients. PLoS ONE. 2016;11:e0153036.
Falk K, Patel H, Swedberg K, Ekman I. Fatigue in patients with chronic heart failure—a burden associated with emotional and symptom distress. Eur J Cardiovasc Nurs. 2009;8:91–6.
Brostrom A, Stromberg A, Dahlstrom U, Fridlund B. Sleep difficulties, daytime sleepiness, and health-related quality of life in patients with chronic heart failure. J Cardiovasc Nurs. 2004;19:234–42.
Wang M-Y, Hung H-L, Tsai P-S. The sleep log and actigraphy: congruency of measurement results for heart failure patients. J Nurs Res. 2011;19:173–80.
Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71:S1–S14.
Maurer MS, Cuddihy P, Weisenberg J, Delisle S, Strong BM, Gao Q, Kachnowski S, Howell J. The prevalence and impact of anergia (lack of energy) in subjects with heart failure and its associations with actigraphy. J Card Fail. 2009;15:145–51.
Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E, NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373:2314–24.
Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin-angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail. 2014;2:663–70.
Entresto®.US Prescribing Information Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. 2019. Available online from https://www.novartis.us/sites/www.novartis.us/files/entresto.pdf. Accessed Sept 17 2020.
McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004.
Velazquez EJ, et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539–48.
Lewis EF, Claggett BL, McMurray JJV, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD, Swedberg K. Health-related quality of life outcomes in PARADIGM-HF. Circ Heart Fail. 2017;10:1–10.
Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T, McMurray JJV, Solomon SD. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA Cardiol. 2018;3:498–505.
Hastings PC, Vazir A, O’Driscoll DM, Morrell MJ, Simonds AK. Symptom burden of sleep-disordered breathing in mild-to-moderate congestive heart failure patients. Eur Respir J. 2006;27:748.
Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55.
Spertus JA, Jones PG, Kim J, Globe D. Validity, reliability, and responsiveness of the Kansas City Cardiomyopathy Questionnaire in anemic heart failure patients. Qual Life Res. 2008;17:291–8.
US Food and Drug Administration. Framework for FDA’s real-world evidence program. 2018. https://www.fda.gov/media/120060/download. Accessed 25 Jan 2019.
Lobelo F, Young DR, Sallis R, Garber MD, Billinger SA, Duperly J, Hutber A, Pate RR, Thomas RJ, Widlansky ME, McConnell MV, Joy EA, American Heart Association Physical Activity Committee of the Council on Lifestyle, and Cardiometabolic Health; Council on Epidemiology, and Prevention; Council on Clinical Cardiology; Council on Genomic, and Precision Medicine; Council on Cardiovascular Surgery, and Anesthesia;, and Stroke Council. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495–e522.
Gresham G, Schrack J, Gresham LM, Shinde AM, Hendifar AE, Tuli R, Rimel BJ, Figlin R, Meinert CL, Piantadosi S. Wearable activity monitors in oncology trials: current use of an emerging technology. Contemp Clin Trials. 2018;64:13–211.
Hickey AM, Freedson PS. Utility of consumer physical activity trackers as an intervention tool in cardiovascular disease prevention and treatment. Prog Cardiovasc Dis. 2016;58:613–9.
Vetrovsky T, Siranec M, Parenica J, Griva M, Stastny J, Precek J, Pelouch R, Bunc V, Linhart A, Belohlavek J. Effect of a 6-month pedometer-based walking intervention on functional capacity in patients with chronic heart failure with reduced (HFrEF) and with preserved (HFpEF) ejection fraction: study protocol for two multicenter randomized controlled trials. J Transl Med. 2017;15:153.
Harding P, Holland AE, Delany C, Hinman RS. Do activity levels increase after total hip and knee arthroplasty? Clin Orthop Relat Res. 2014;472:1502–11.
Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A Jr, Coleman J, Lee-Chiong T, Pancer J. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–29.
Liebzeit D, Phelan C, Moon C, Brown R, Bratzke L. Rest–activity patterns in older adults with heart failure and healthy older adults. J Aging Phys Act. 2017;25:116–22.
Hetzenecker A, Escourrou P, Kuna ST, Series F, Lewis K, Birner C, Pfeifer M, Arzt M. Treatment of sleep apnea in chronic heart failure patients with auto-servo ventilation improves sleep fragmentation: a randomized controlled trial. Sleep Med. 2016;17:25–31.
Acknowledgements
Visualization of data was provided by Jonas Dorn of Novartis (Basel, Switzerland) and expertise regarding sleep testing was provided by Michael Coppola, MD, of NovaSom (Glen Burnie, MD, USA). Medical writing and editorial assistance were provided by Janel Torsiello, PharmD, and Traci Stuve, MA, of ApotheCom (Yardley, PA, USA), funded by Novartis Pharmaceuticals Corporation.
Funding
The AWAKE-HF study was sponsored and funded by Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA) and was designed through a collaboration of the sponsor and the study’s steering committee. The sponsor provided consultant compensation to the steering committee member authors for their trial-related activities.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Raj M. Khandwalla has served as a consultant for Novartis Pharmaceuticals Corporation and has received a research grant from General Electric. Kade Birkeland has served as a consultant for Novartis Pharmaceuticals Corporation. J. Thomas Heywood has served as a consultant for Actelion Pharmaceuticals, Medtronic, and Abbott; was a speaker for Actelion Pharmaceuticals, Medtronic, Abbott, Otsuka, and Novartis Pharmaceuticals Corporation; has received grant funding from Medtronic, Abbott, and Impedimed; and has received fellowship grant support from Abbott. Robert L. Owens has received honoraria and travel reimbursements from ResMed, LLC, and Itamar Medical and has served as a consultant for Novartis Pharmaceuticals Corporation. Steven Steinhubl has received financial compensation from Novartis Pharmaceuticals Corporation for serving as an adviser. Emmanuel Fombu was an employee of Novartis Pharmaceuticals at the time of this study. Daniel Grant is an employee of Novartis Pharmaceuticals.
Ethics approval
Not applicable
Consent to participate
All participants in the trial signed an informed consent upon enrollment.
Consent for publication
All co-authors have consented for publication.
Availability of data and materials
Data and materials were made available to the co-investigators.
Code availability
Not applicable.
Author Contributions
All authors contributed to the conception and design of the study and/or data analysis/interpretation, drafting, and/or critical review of the manuscript, and all provided final approval to submit the manuscript for publication. RMK wrote the first draft of the manuscript, and the other authors, as part of the steering committee, revised subsequent drafts. Medical writing and editing assistance was provided by ApotheCom (Yardley, PA, USA), funded by Novartis Pharmaceuticals Corporation. All authors agree to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. A full list of all investigators and institutions involved in the study is provided in Table S1 in the ESM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khandwalla, R.M., Grant, D., Birkeland, K. et al. The AWAKE-HF Study: Sacubitril/Valsartan Impact on Daily Physical Activity and Sleep in Heart Failure. Am J Cardiovasc Drugs 21, 241–254 (2021). https://doi.org/10.1007/s40256-020-00440-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-020-00440-y