Abstract
Background
Inhibition of bromodomain and extra-terminal (BET) proteins can modulate lipoprotein and inflammatory factors that mediate atherosclerosis. The impact of the BET inhibitor, apabetalone, on cardiovascular events is unknown.
Objective
Our objective was to investigate the impact of apabetalone on cardiovascular event rates in a pooled analysis of clinical studies in patients with established coronary artery disease.
Methods
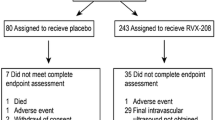
We conducted a pooled analysis of patients (n = 798) with coronary artery disease who participated in clinical trials (ASSERT, ASSURE, SUSTAIN) that evaluated the impact of 3–6 months of treatment with apabetalone on lipid parameters and coronary atherosclerosis. The incidence of major adverse cardiovascular events (death, myocardial infarction, coronary revascularization, hospitalization for cardiovascular causes) in the treatment groups was evaluated.
Results
At baseline, patients treated with apabetalone were more likely to be Caucasian, have a history of dyslipidemia, and be undertreated with ß-blocker and anti-platelet agents. Treatment with apabetalone produced the following dose-dependent changes compared with placebo: increases in apolipoprotein A-I (apoA-I) of up to 6.7% (P < 0.001), increases in high-density lipoprotein cholesterol (HDL-C) of up to 6.5% (P < 0.001), increases in large HDL particles of up to 23.3% (P < 0.001), and decreases in high-sensitivity C-reactive protein (hsCRP) of − 21.1% (P = 0.04). Apabetalone treatment did not affect atherogenic lipoproteins compared with placebo. Patients treated with apabetalone experienced fewer major adverse cardiovascular events than those treated with placebo (5.9 vs. 10.4%; P = 0.02), a finding that was more prominent in patients with diabetes (5.4 vs. 12.7%; P = 0.02), with baseline HDL-C < 39 mg/dl (5.5 vs. 12.8%; P = 0.01), or with elevated hsCRP levels (5.4 vs. 14.2%; P = 0.02).
Conclusion
Pooled analysis of short-term studies demonstrated fewer cardiovascular events among patients treated with the BET protein inhibitor, apabetalone, than among those treated with placebo. BET protein inhibition warrants further investigation as a novel approach to cardiovascular risk reduction.


Similar content being viewed by others
References
Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46:1225–8.
Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26.
Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8.
Catena C, Novello M, Lapenna R, et al. New risk factors for atherosclerosis in hypertension: focus on the prothrombotic state and lipoprotein(a). J Hypertens. 2005;23:1617–31.
Meslamani J, Smith SG, Sanchez R, Zhou MM. Structural features and inhibitors of bromodomains. Drug Discov Today Technol. 2016;19:3–15.
McLure KG, Gesner EM, Tsujikawa L, et al. RVX-208, an inducer of ApoA-I in humans, is a BET bromodomain antagonist. PLoS One. 2013;8:e83190.
Wasiak S, Gilham D, Tsujikawa LM et al. Downregulation of the complement cascade in vitro, in mice and in patients with cardiovascular disease by the BET protein inhibitor apabetalone (RVX-208). J Cardiovasc Transl Res. 2017.
Wasiak S, Gilham D, Tsujikawa LM, et al. Data on gene and protein expression changes induced by apabetalone (RVX-208) in ex vivo treated human whole blood and primary hepatocytes. Data Brief. 2016;8:1280–8.
Bailey D, Jahagirdar R, Gordon A, et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol. 2010;55:2580–9.
Di Bartolo BA, Scherer DJ, Nicholls SJ. Inducing apolipoprotein A-I synthesis to reduce cardiovascular risk: from ASSERT to SUSTAIN and beyond. Arch Med Sci: AMS. 2016;12:1302–7.
Nicholls SJ, Gordon A, Johansson J, et al. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J Am Coll Cardiol. 2011;57:1111–9.
Nicholls SJ, Puri R, Wolski K, et al. Effect of the BET protein inhibitor, RVX-208, on progression of coronary atherosclerosis: results of the phase 2b, randomized, double-blind, multicenter, ASSURE Trial. Am J Cardiovasc Drug. 2016;16:55–65.
Gilham D, Wasiak S, Tsujikawa LM, et al. RVX-208, a BET-inhibitor for treating atherosclerotic cardiovascular disease, raises ApoA-I/HDL and represses pathways that contribute to cardiovascular disease. Atherosclerosis. 2016;247:48–57.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The studies were funded by Resverlogix.
Conflicts of interest
SJN has received research support from AstraZeneca, Amgen, Anthera, Eli Lilly, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron, and LipoScience and is a consultant for AstraZeneca, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, and Boehringer Ingelheim. KKR reports grants and/or personal fees from Pfizer, MSD, Astra Zeneca, Sanofi, Aegerion, Regeneron, Abbvie, Kowa, Cerenis, Medicines Company, Lilly, Esperion, Amgen, Cipla, Algorithm, Takeda, Boehringer Ingelheim, and Novo Nordisk within the last 12 months outside of the submitted work. GGS, through his institution, has received research support from Cerenis, The Medicines Company, Resverlogix, Roche, and Sanofi. JOJ, AG, MS, CH, EK, and NW are employees of Resverlogix. SWK has no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nicholls, S.J., Ray, K.K., Johansson, J.O. et al. Selective BET Protein Inhibition with Apabetalone and Cardiovascular Events: A Pooled Analysis of Trials in Patients with Coronary Artery Disease. Am J Cardiovasc Drugs 18, 109–115 (2018). https://doi.org/10.1007/s40256-017-0250-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-017-0250-3