Abstract
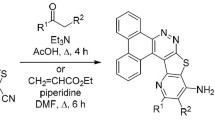
Novel 3-substituted pyridothienopyrimidine derivatives have been synthesized via the reaction of 2-{7,9-dimethyl-4-oxopyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-3(4H)-yl}acetohydrazide(5) with a variety of active reagents and chemicals. Structures of the newly synthesized compounds were established based on spectral data. The resulting pyridothienopyrimidine derivatives were evaluated for their possible antimicrobial activity and some of them represent a new class of potentially antimicrobial compounds, especially compounds 9 and 18 which displayed the highest activity against Gram-positive bacteria, Gram-negative bacteria, and Fungi in MIC range of 0.12―1.95 μg/mL.
Similar content being viewed by others
References
Vitaku E., Smith D. T., Njardarson J. T., J. Med. Chem., 2014, 57(24), 10257
Khattab A. F., El-Sakka I. A., Yassin S. M., El-Essawy F. A. G., Sulfur. Lett., 1995, 19(1), 23
Bousquet E., Romeo G., Guerrera F., Caruso A., Amico-Roxas M., Farmaco 1985, 40(11), 869
Badr M. Z. A., Mahgoub S. A., Abdel-Latif F. F., Abd El-Hafez A. A. A., Phosphorus, Sulfur SiliconRelat. Elem., 1991, 55(1-4), 175
Bernardino A. M., da Silva PinheiroL. C., Rodrigues C. R., Loureiro N. I., Castro H. C., Lanfredi-Rangel A., Sabatini-Lopes J., Borges J. C., Carvalho J. M., Romeiro G. A., Ferreira V. F., Bioorg. Med. Chem., 2006, 14(16), 5765
Al-Trawneh S. A., El-Abadelah M. M., Zahra J. A., Al-Taweel S. A., Zani F., Incerti M., Cavazzoni A., Vicini, P., Bioorg. Med. Chem. 2011, 19(8), 2541
Abdel-Rahman A. E., Bakhite E. A., Al-Taifi E. A., J. Chin. Chem. Soc., 2002, 49(2), 223
Leistner S., Wagener G., Guestscharo M., Glusa E., Pharmazie 1986, 41(1), 54
Bousquent E., Romero G., Guerrera F., Caruso A., Roxas M. A., Farmaco Ed. Sci., 1985, 40, 869
Zhao C., Tovar C., Yin X., Xu Q., Todorov I. T., Vassilev L. T., Chen L., Bioorg. Med. Chem. Lett. 2009, 19(2), 319
Abdelriheem N. A., Ahmad S. A. K., Abdelhamid A. O., Molecules, 2015, 20(1), 822
Bing S., Xiu’e Y., Jin Z., Jian H., Yue X., Furong Z., Jinhui W., Guoqing W., Chun H., Chem. Res. Chinese Universities 2015, 31(6), 936
Litvinov V. P., Dotsenko V. V., Krivokolysko S. G., NaukaMoscow, 2006, 72
Hossan A. S. M., Abu-Melha H. M. A., Al-Omar M. A., Amr A. E. E., Molecules, 2012, 17(11), 13642
Bakhite E. A., Abdel-Rahman A. E., Mohamed O. S., Thabet E. A., Bull. Korean Chem. Soc., 2002, 23(12), 1709
Rateb N. M., Abdelaziz S. H., Zohdi H. F., J. Sulfur Chem., 2011, 32(4), 345
Gaber H. M., Elgemeie G. E. H., Ouf S. A., Sherif S. M., Heteroatom Chem., 2005, 16(4), 298
El-Essawy F. A., Nucleos. Nucleot. Nucl., 2005, 24(8), 1265
El-Essawy F. A., Chem. Heterocycl. Comp., 2009, 45(7), 837
El-Essawy F. A., Hawatta M. A., Abdel-Megied A. E., El-Sherbeny D. A., Chem. Heterocycl. Comp., 2010, 46(3), 325
El-Essawy F. A., J. Heterocyclic. Chem., 2010, 47(2), 318
El-Essawy F. A., Synthetic Commun., 2010, 40(6), 877
Peinador C., Ojea V., Quintela J. M., J. Heterocycl. Chem., 1992, 29(7), 1693
Quintela J. M., Peinador C., Veiga C., Gonzales L., Botana L. M., Alfonso A., Riguera R., Bioorg. Med. Chem. 1998, 6(10), 1911
El-Essawy F. A., El-Sayed W. A., J. Heterocyclic. Chem., 2013, 50(S1), E1
Clinical and Laboratory Standards Institute, Performance Standards for Antimicrobial Susceptibility Testing, Twenty-Fourth Informatio- nal Supplement(M100-S24), Wayne, 2014, 34(1), 1
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Deanship of Scientific Research at Prince Sattam Bin Abdulaziz University(No.2015/01/4713).
Electronic supplementary material
40242_2016_6218_MOESM1_ESM.pdf
Studies on synthesis of novel 3-substituted pyridothienopyrimidine derivatives with biological evaluation as antimicrobial agents
Rights and permissions
About this article
Cite this article
El-Essawy, F.A., Boshta, N.M., El-Sawaf, A.K. et al. Synthesis of novel 3-substituted pyridothienopyrimidine derivatives with biological evaluation as antimicrobial agents. Chem. Res. Chin. Univ. 32, 967–972 (2016). https://doi.org/10.1007/s40242-016-6218-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-016-6218-z