Abstract
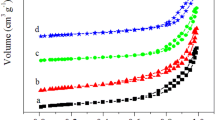
SiO2 with different nanostructures, namely hexagonal mesoporous silica(HMS), and three unordered commercial silica, were used as supports to fabricate silver catalysts using an incipient wetness impregnation method. It was found that Ag/HMS catalyst showed a high catalytic activity. Next, the HMS support was calcined at different temperatures before impregnation of AgNO3. The effect of calcination temperature of HMS support was investigated in terms of structure and catalytic activity of Ag catalysts. The support and catalysts were characterized by N2 adsorption-desorption isotherms, Thermogravimetric-differential thermal analyzer, X-ray diffraction, H2-temperature program reduction and transmission electron microscopy. The results showed that calcination of HMS at an appropriate temperature(750 °C) before catalyst preparation would benefit the formation of highly dispersive small sized Ag particles on the HMS support and markedly enhance the catalytic activity of Ag/HMS catalyst toward CO oxidation.
Similar content being viewed by others
References
Jernigan G. G., Somorjai G. A., J. Catal., 1994, 147, 567
Cuong N. D., Khieu D. Q., Hoa T. T., Quang D. T., Viet P. H., Lam T. D., Hoa N. D., Hieu N. V., Mater. Res. Bull., 2015, 68, 302
Kageyama S., Sugano Y., Hamaguchi Y., Kugai J., Ohkubo Y., Seino S., Nakagawa T., Ichikawa S., Yamamoto T. A., Mater. Res. Bull., 2013, 48, 1347
Zhang X. D., Dong H., Gu Z. J., Wang G., Zuo Y. H., Wang Y. G., Cui L. F., Chem. Eng. J., 2015, 269, 94
Dong Q., Yin S., Guo C. S., Wu X. Y., Kimura T., Sato T., Mater. Res. Bull., 2013, 48, 4989
Xu X. Y., Li J. J., Hao Z. P., Zhao W., Hu C., Mater. Res. Bull., 2006, 41, 406
Qu Z. P., Zhang X. D., Yu F. L., Liu X. C., Fu Q., J. Catal., 2015, 321, 113
Zhang X. D., Qu Z. P., Yu F. L., Wang Y., J. Catal., 2013, 297, 264
Menacherry P. V., Fernandez-Garcia M., Haller G. L., J. Catal., 1997, 166, 75
Bore M. T., Pham H. N., Ward T. L., Datye A. K., Chem. Commun., 2004, 2620
Zhang X. D., Qu Z. P., Jia J. J., Wang Y., Powder Technol., 2012, 230, 212
Qu Z. P., Zhang X. D., Yu F. L., Jia J. X., Micro. Meso. Mater., 2014, 188, 1
Qu Z. P., Huang W. X., Zhou S. T., Zheng H., Liu X. M., Cheng M. J., Bao X. H., J. Catal., 2005, 234, 33
Bagshaw S. A., Prouzet E., Pinnavaia T. J., Science, 1995, 269, 1242
Li X. F., Gao H. X., Jin G. J., Chen L., Ding L., Yang H. Y., Chen Q. L., J. Mol. Struct., 2008, 872, 10
Chiranjeevi T., Kumar P., Maity S. K., Rana M. S., Murali Dhar G., Prasada Rao T. S. R., Micro. Meso. Mater., 2001, 44, 547
Sing K. S. W., Everett D. H., Haul R. A. W., Moscow L., Pierotti R. A., Ronquerol J., Pure Appl. Chem., 1985, 57, 603
Zhang X. D., Qu Z. P., Li X. Y., Zhao Q. D., Zhang X., Quan X., Mater. Lett., 2011, 65, 1892
Yu J., Mao D. S., Guo Q. S., Han L. P., Lu G. Z., Acta Phys. Chim. Sin., 2012, 28, 667
Qu Z. P., Zhang X. D., Lv Y., Quan X., Fu Q., J. Nanosci. Nanotech., 2013, 13, 4573
Montesinos-Castellanos A., Zepeda T. A., Micro. Meso. Mater., 2008, 113, 146
Zhang X. D., Qu Z. P., Li X. Y., Zhao Q. D., Zhang X., Quan X., Catal. Commun., 2011, 16, 11
Citrin P. H., Wertheim C. K., Baer T., Phys. Rev. B, 1983, 27, 3160
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China(Nos.21507086, 11305099, 41473108), the Shanghai Sailing Program, China(No.14YF1409900) and the Hujiang Foundation Research Base Program, China(Nos.B14003, D14004).
Rights and permissions
About this article
Cite this article
Zhang, X., Dong, H., Zhao, D. et al. Effect of support calcination temperature on Ag structure and catalytic activity for CO oxidation. Chem. Res. Chin. Univ. 32, 455–460 (2016). https://doi.org/10.1007/s40242-016-5377-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-016-5377-2