Abstract
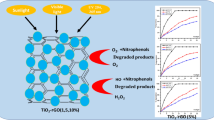
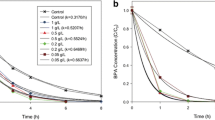
In this photocatalytic study, removal of bisphenol-A from aqueous solution was studied using the GF/Fe-TiO2-CQD composite. Due to its health and environmental effects, this compound should be disposed of sources that are mainly industrial wastewater. The phis-chemical properties of the composite were determined by traditional analyzes of EF-SEM, EDX, BET, XRD, FTIR and DRS. In this study, different ratios of CQD in the composite (1.5, 4.5 and 7.5 wt%), pH, and bisphenol-A concentration as variable parameters were investigated. All analyzes, EF-SEM, EDX, BET, XRD, FTIR, show that the GF/Fe-TiO2-CQD composite is well coated on glass fibers (GF) and all the elements in the catalyst are present. On the other hand, DRS analysis showed that CQD reduces the band gap of Fe-TiO2 from 2.96 eV to 2.91 eV, it was 3.10 eV for TiO2. Among different catalysts, GF/Fe-TiO2-CQD4.5wt% has the best performance. The results showed that for GF/Fe-TiO2-CQD4.5wt%, optimum for the process was at pH = 6 in low concentration of bisphenol-A. The first order model for the photocatalytic degradation process were well studied. In addition, GF/Fe-TiO2-CQD4.5wt% showed that it can be used many times with a minimal reduction in performance. As a result, the GF/Fe-TiO2-CQD4.5wt% composite can successfully remove bisphenol-A form in synthetic aqueous solution. However, it is necessary to further studies to applied that for real water source in water and wastewater treatment plants.









Similar content being viewed by others
References
Vakili M, Mojiri A, Kindaichi T, Cagnetta G, Yuan J, Wang B, et al. Cross-linked chitosan/zeolite as a fixed-bed column for organic micropollutants removal from aqueous solution, optimization with RSM and artificial neural network. J. Environ. Manage. [internet] 2019;250:109434. Available from: https://doi.org/10.1016/j.jenvman.2019.109434, 2019.
Hacıosmanoğlu GG, Doğruel T, Genç S, Oner ET, Can ZS. Adsorptive removal of bisphenol a from aqueous solutions using phosphonated Levan. J Hazard Mater. 2019;374:43–9.
Zhou Y, Lu J, Liu Q, Chen H, Liu Y, Zhou Y. A novel hollow-sphere cyclodextrin nanoreactor for the enhanced removal of bisphenol a under visible irradiation. J. Hazard. Mater. [internet] 2020;384:121267. Available from: https://doi.org/10.1016/j.jhazmat.2019.121267, 2020.
Li X, Zhou M, Jia J, Ma J, Jia Q. Design of a hyper-crosslinked β-cyclodextrin porous polymer for highly efficient removal toward bisphenol a from water. Sep. Purif. Technol. [internet] 2018;195:130–7. Available from: https://doi.org/10.1016/j.seppur.2017.12.007, 2018.
Chen ZH, Liu Z, Hu JQ, Cai QW, Li XY, Wang W, et al. β-Cyclodextrin-modified graphene oxide membranes with large adsorption capacity and high flux for efficient removal of bisphenol a from water. J. Memb. Sci. [internet] 2020;595:117510. Available from: https://doi.org/10.1016/j.memsci.2019.117510, 2020.
Fang Z, Hu Y, Cheng J, Chen Y. Continuous removal of trace bisphenol a from water by high efficacy TiO2 nanotube pillared graphene-based macrostructures in a photocatalytically fluidized bed. Chem. Eng. J. [internet] 2019;372:581–9. Available from: https://doi.org/10.1016/j.cej.2019.04.129, 2019.
Saroyan HS, Bele S, Giannakoudakis DA, Samanidou VF, Bandosz TJ, Deliyanni EA. Degradation of endocrine disruptor, bisphenol-A, on an mixed oxidation state manganese oxide/modified graphite oxide composite: A role of carbonaceous phase. J. Colloid Interface Sci. [Internet] 2019;539:516–24. Available from: http://www.sciencedirect.com/science/article/pii/S0021979718315297
López-Ramón MV, Ocampo-Pérez R, Bautista-Toledo MI, Rivera-Utrilla J, Moreno-Castilla C, Sánchez-Polo M. Removal of bisphenols A and S by adsorption on activated carbon clothes enhanced by the presence of bacteria. Sci. Total Environ. [Internet] 2019;669:767–76. Available from: http://www.sciencedirect.com/science/article/pii/S0048969719311076
Choi Y-K, Kan E. Effects of pyrolysis temperature on the physicochemical properties of alfalfa-derived biochar for the adsorption of bisphenol A and sulfamethoxazole in water. Chem Int. 2019;218:741–8 Available from: http://www.sciencedirect.com/science/article/pii/S0045653518322586.
Zhou L, Richard C, Ferronato C, Chovelon J-M, Sleiman M. Investigating the performance of biomass-derived biochars for the removal of gaseous ozone, adsorbed nitrate and aqueous bisphenol A. Chem. Eng. J. [Internet] 2018;334:2098–104. Available from: http://www.sciencedirect.com/science/article/pii/S1385894717320673
Bhadra BN, Lee JK, Cho CW, Jhung SH. Remarkably efficient adsorbent for the removal of bisphenol a from water: bio-MOF-1-derived porous carbon. Chem Eng J. 2018;343:225–34.
Lin Q, Wu Y, Jiang X, Lin F, Liu X, Lu B. Removal of bisphenol A from aqueous solution via host-guest interactions based on beta-cyclodextrin grafted cellulose bead. Int. J. Biol. Macromol. [Internet] 2019;140:1–9. Available from: http://www.sciencedirect.com/science/article/pii/S014181301933329X
Luo Z, Chen H, Wu S, Yang C, Cheng J. Enhanced removal of bisphenol a from aqueous solution by aluminum-based MOF/sodium alginate-chitosan composite beads. Chemosphere [internet] 2019;237:124493. Available from: https://doi.org/10.1016/j.chemosphere.2019.124493, 2019.
Chen S, Chi M, Yang Z, Gao M, Wang C, Lu X. Carbon dots/Fe3O4 hybrid nanofibers as efficient peroxidase mimics for sensitive detection of H2O2 and ascorbic acid. Inorg Chem Front. 2017;4:1621–7.
Li G, Zhang X, Sun J, Zhang A, Liao C. Effective removal of bisphenols from aqueous solution with magnetic hierarchical rattle-like co/Ni-based LDH. J. Hazard. Mater. [internet] 2020;381:120985. Available from: https://doi.org/10.1016/j.jhazmat.2019.120985, 2020.
Baran W, Adamek E, Sobczak A, Makowski A. Photocatalytic degradation of sulfa drugs with TiO2, Fe salts and TiO2/FeCl3 in aquatic environment-kinetics and degradation pathway. Appl Catal B Environ. 2009;90:516–25.
Xu L, Yang L, Johansson EMJ, Wang Y, Jin P. Photocatalytic activity and mechanism of bisphenol a removal over TiO2−x/rGO nanocomposite driven by visible light. Chem Eng J [Internet] 2018;350:1043–55. Available from: http://www.sciencedirect.com/science/article/pii/S1385894718310842
Nguyen TB, Huang CP, Doong R An. Photocatalytic degradation of bisphenol a over a ZnFe 2 O 4 /TiO 2 nanocomposite under visible light. Sci. Total environ. [internet] 2019;646:745–56. Available from: https://doi.org/10.1016/j.scitotenv.2018.07.352, 2019.
Aghamali A, Khosravi M, Hamishehkar H, Modirshahla N, Behnajady MA. Preparation of novel high performance recoverable and natural sunlight-driven nanocomposite photocatalyst of Fe3O4/C/TiO2/N-CQDs. Mater. Sci. Semicond. Process. [internet] 2018;87:142–54. Available from: https://doi.org/10.1016/j.mssp.2018.07.018, 2018.
Jafari AJ, Mahrooghi M, Moslemzadeh M. Removal of Escherichia coli from synthetic turbid water using titanium tetrachloride and zirconium tetrachloride as coagulants. Desalin Water Treat. 2019;163:358–65.
Gu Y, Yperman J, Carleer R, D’Haen J, Maggen J, Vanderheyden S, et al. Adsorption and photocatalytic removal of ibuprofen by activated carbon impregnated with TiO2 by UV–Vis monitoring. Chemosphere [internet] 2019;217:724–31. Available from: https://doi.org/10.1016/j.chemosphere.2018.11.068, 2019.
Xue G, Liu H, Chen Q, Hills C, Tyrer M, Innocent F. Synergy between surface adsorption and photocatalysis during degradation of humic acid on TiO2/activated carbon composites. J. Hazard. Mater. [internet] 2011;186:765–72. Available from: https://doi.org/10.1016/j.jhazmat.2010.11.063, 2011.
Law M, Greene LE, Johnson JC, Saykally R, Yang P. Nanowire dye-sensitized solar cells. Mater Sustain Energy A Collect Peer-Reviewed Res Rev Artic from Nat Publ Gr. 2010;4:75–9.
Xu X, Liu R, Cui Y, Liang X, Lei C, Meng S, et al. PANI/FeUiO-66 nanohybrids with enhanced visible-light promoted photocatalytic activity for the selectively aerobic oxidation of aromatic alcohols. Appl. Catal. B environ. [internet] 2017;210:484–94. Available from: https://doi.org/10.1016/j.apcatb.2017.04.021, 2017.
Benyamina I, Manseri K, Mansour M, Benalioua B, Bentouami A, Boury B. New bi 2 O 3 -ZnO composite deposited on glass wool. Effect of the synthesis method on photocatalytic efficiency under visible light. Appl. Surf. Sci. [internet] 2019;483:859–69. Available from: https://doi.org/10.1016/j.apsusc.2019.03.310, 2019.
Nishimura A, Zhao X, Hayakawa T, Ishida N, Hirota M, Hu E. Impact of overlapping Fe/TiO2 prepared by sol-gel and dip-coating process on CO2 reduction. Int. J. Photoenergy 2016;2016.
Tian L, Xing L, Shen X, Li Q, Ge S, Liu B, et al. Visible light enhanced Fe–I–TiO2 photocatalysts for the degradation of gaseous benzene. Atmos. Pollut. Res. [Internet] 2020;11:179–85. Available from: http://www.sciencedirect.com/science/article/pii/S1309104219304799
Chen Q, Chen L, Qi J, Tong Y, Lv Y, Xu C, et al. Photocatalytic degradation of amoxicillin by carbon quantum dots modified K2Ti6O13 nanotubes: Effect of light wavelength. Chinese Chem. Lett. [Internet] 2019;Available from: https://doi.org/10.1016/j.cclet.2019.03.002
Gu L, Chen Z, Sun C, Wei B, Yu X. Photocatalytic degradation of 2, 4-dichlorophenol using granular activated carbon supported TiO2. Desalination. 2010;263:107–12.
Lee JC, Kim MS, Kim BW. Removal of paraquat dissolved in a photoreactor with TiO2 immobilized on the glass-tubes of UV lamps. Water Res. 2002;36:1776–82.
Dehghani MH, Kamalian S, Shayeghi M, Yousefi M, Heidarinejad Z, Agarwal S, et al. High-performance removal of diazinon pesticide from water using multi-walled carbon nanotubes. Microchem. J. [internet] 2019;145:486–91. Available from: https://doi.org/10.1016/j.microc.2018.10.053, 2019.
Kaur T, Sraw A, Wanchoo RK, Toor AP. Solar assisted degradation of carbendazim in water using clay beads immobilized with TiO2 & Fe doped TiO2. Sol. Energy [internet] 2018;162:45–56. Available from: https://doi.org/10.1016/j.solener.2017.11.033, 2018.
Lin S, Zhang X, Sun Q, Zhou T, Lu J. Fabrication of solar light induced Fe-TiO2 immobilized on glass-fiber and application for phenol photocatalytic degradation. Mater. Res. Bull. [internet] 2013;48:4570–5. Available from: https://doi.org/10.1016/j.materresbull.2013.07.063, 2013.
Wang CY, Böttcher C, Bahnemann DW, Dohrmann JK. A comparative study of nanometer sized Fe(III)-doped TiO2 photocatalysts: synthesis, characterization and activity. J Mater Chem. 2003;13:2322–9.
Chen P, Wang F, Chen ZF, Zhang Q, Su Y, Shen L, et al. Study on the photocatalytic mechanism and detoxicity of gemfibrozil by a sunlight-driven TiO2/carbon dots photocatalyst: the significant roles of reactive oxygen species. Appl. Catal. B environ. [internet] 2017;204:250–9. Available from: https://doi.org/10.1016/j.apcatb.2016.11.040, 2017.
Jafari AJ, Moslemzadeh M. Synthesis of Fe-doped TiO 2 for Photocatalytic processes under UV-visible light : effect of preparation methods on crystal size — a systematic review study. COMMENTSONINORGANIC Chem 2020;
Taghavi K, Naghipour D, Mohagheghian A, Moslemzadeh M, Taghavi K, Moslemzadeh M, et al. Adsorption of chromium(VI) from aqueous solution by Artist’s bracket fungi. Water Sci. Technol. [internet] 2017;158:207–15. Available from: https://doi.org/10.1080/01496395.2017.1299179, 2017.
Pourkarim S, Ostovar F, Mahdavianpour M, Moslemzadeh M. Adsorption of chromium(VI) from aqueous solution by Artist’s bracket fungi. Sep. Sci. Technol. [internet] 2017;52:1733–41. Available from: https://doi.org/10.1080/01496395.2017.1299179, 2017.
Ibrahim SA, Anwar MK, Ainuddin AR, Hariri A, Rus AZM, Kamdi Z, et al. Synthesis and characterization of visible light active Fe-TiO2 using hydrothermal method. Int J Integr Eng [Internet] 2019;11:80–5. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85074842080&doi=10.30880%2Fijie.2019.11.05.011&partnerID=40&md5=cfce9a4ca5be1bc632af2cc125fc748b
Esrafili A, Bagheri S, Kermani M, Gholami M, Moslemzadeh M. Simultaneous adsorption of heavy metal ions (Cu2+ and Cd2+) from aqueous solutions by magnetic silica nanoparticles (Fe3O4@SiO2) modified using edta. Desalin Water Treat. 2019;158:207–15.
Li K, Wang H, Pan C, Wei J, Xiong R, Shi J. Enhanced photoactivity of Fe + N Codoped anatase-rutile TiO 2 nanowire film under visible light irradiation. Int J Photoenergy. 2012;2012:1–8.
Arias LMF, Duran AA, Cardona D, Camps E, Gómez ME, Zambrano G. Effect of annealing treatment on the photocatalytic activity of TiO2 thin films deposited by dc reactive magnetron sputtering. J Phys Conf Ser. 2015;614:012008.
Yadav HM, Kolekar TV, Pawar SH, Kim J-S. Enhanced photocatalytic inactivation of bacteria on Fe-containing TiO2 nanoparticles under fluorescent light. J Mater Sci Mater Med. 2016;27:57.
Aghamali A, Khosravi M, Hamishehkar H, Modirshahla N, Behnajady MA. Preparation of novel high performance recoverable and natural sunlight-driven nanocomposite photocatalyst of Fe3O4/C/TiO2/N-CQDs. Mater Sci Semicond Process [Internet] 2018;87:142–54. Available from: http://www.sciencedirect.com/science/article/pii/S136980011732560X
Jafari AJ, Moslemzadeh M. Synthesis of Fe-doped TiO 2 for Photocatalytic processes under UV-visible light : effect of preparation methods on crystal size — a systematic review study. Commentsoninorganic Chem. [internet] 2020;00:1–20. Available from: https://doi.org/10.1080/02603594.2020.1821674, 2020.
Zhang J, Kuang M, Wang J, Liu R, Xie S, Ji Z. Fabrication of carbon quantum dots/TiO2/Fe2O3 composites and enhancement of photocatalytic activity under visible light. Chem. Phys. Lett. [internet] 2019;730:391–8. Available from: https://doi.org/10.1016/j.cplett.2019.06.011, 2019.
Jonidi A, Moslemzadeh M. Adsorption of lead ( Pb 2 + ) onto salicylic acid-methanol modified steel slag from aqueous solution : a cost analysis. 2020;198:200–10.
Zhao X, Du P, Cai Z, Wang T, Fu J, Liu W. Photocatalysis of bisphenol a by an easy-settling titania/titanate composite: effects of water chemistry factors, degradation pathway and theoretical calculation. Environ Pollut [Internet] 2018;232:580–90. Available from: http://www.sciencedirect.com/science/article/pii/S0269749117313799
Ahamad T, Naushad M, Ruksana, Alhabarah AN, Alshehri SM. N/S doped highly porous magnetic carbon aerogel derived from sugarcane bagasse cellulose for the removal of bisphenol-a. Int. J. Biol. Macromol. [internet] 2019;132:1031–8. Available from: https://doi.org/10.1016/j.ijbiomac.2019.04.004, 2019.
Frontistis Z, Daskalaki VM, Katsaounis A, Poulios I, Mantzavinos D. Electrochemical enhancement of solar photocatalysis: degradation of endocrine disruptor bisphenol-a on Ti/TiO2 films. Water Res [Internet] 2011;45:2996–3004. Available from: http://www.sciencedirect.com/science/article/pii/S0043135411001412
Yari K, Seidmohammadi A, Khazaei M, Bhatnagar A, Leili M. A comparative study for the removal of imidacloprid insecticide from water by chemical-less UVC, UVC/TiO2 and UVC/ZnO processes. J Environ Heal Sci Eng. 2019;17:337–51.
Zhou G, Cao Y, Jin Y, Wang C, Wang Y, Hua C, et al. Novel selective adsorption and photodegradation of BPA by molecularly imprinted sulfur doped nano-titanium dioxide. J. Clean. Prod. [internet] 2020;274:122929. Available from: https://doi.org/10.1016/j.jclepro.2020.122929, 2020.
Armaković SJ, Grujić-Brojčin M, Šćepanović M, Armaković S, Golubović A, Babić B, et al. Efficiency of La-doped TiO2 calcined at different temperatures in photocatalytic degradation of β-blockers. Arab J Chem. 2019;12:5355–69.
Mahdavianpour M, Ildari S, Ebrahimi M, Moslemzadeh M. Decolorization and Mineralization of Methylene Blue in Aqueous Solutions by Persulfate / Fe 2 + Process. 2020;42:244–51.
Kujlu R, Moslemzadeh M, Rahimi S, Aghayani E, Ghanbari F, Mahdavianpour M. Selecting the best stabilization/solidification method for the treatment of oil-contaminated soils using simple and applied best-worst multi-criteria decision-making method. Environ. Pollut. [internet] 2020;263:114447. Available from: https://doi.org/10.1016/j.envpol.2020.114447, 2020.
Liu CM, Diao ZH, Huo WY, Kong LJ, Du JJ. Simultaneous removal of Cu2+ and bisphenol a by a novel biochar-supported zero valent iron from aqueous solution: synthesis, reactivity and mechanism. Environ. Pollut. [internet] 2018;239:698–705. Available from: https://doi.org/10.1016/j.envpol.2018.04.084, 2018.
Jaseela PK, Shamsheera KO, Joseph A. Mesoporous Titania-silica nanocomposite as an effective material for the degradation of Bisphenol a under visible light. J Saudi Chem Soc. 2020;24:651–62.
Taghavi K, Naghipour D, Mohagheghian A, Moslemzadeh M. Photochemical degradation of 2,4-dichlorophenol in aqueous solutions by Fe2+/ Peroxydisulfate/ UV process. Int J Eng [Internet] 2017;30:15–22. Available from: http://www.ije.ir/Vol30/No1/A/3.pdf
Acknowledgments
The present study was adapted from the PhD thesis of Mehrdad Moslemzadeh at Iran University of Medical Sciences.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Funding
The present project was financially funded by grant number 98–4–2-16,676 from Iran University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Ahmad Jonidi Jafari: Investigation, Writing - original draft Writing – review & editing.
Roshanak Rezaei Kalantary; Ali Esrafili: Writing – review & editing.
Mehrdad Moslemzadeh: Supervision, Writing – review & editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jafari, A.J., Kalantary, R.R., Esrafili, A. et al. Photo-catalytic degradation of bisphenol-a from aqueous solutions using GF/Fe-TiO2-CQD hybrid composite. J Environ Health Sci Engineer 19, 837–849 (2021). https://doi.org/10.1007/s40201-021-00651-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-021-00651-8