Abstract
Purpose
Diabetes accelerates peripheral, distal symmetric polyneuropathy, small fiber predominant neuropathy, radiculoplexopathy, and autonomic neuropathy. This study investigated the neuroprotective effects of gallic acid and myricetin-rich Labisia pumila extract in a diabetic neuropathy rat model and evaluated the neuropathy correlationship with serum inflammatory biomarkers.
Methods
Thirty male rats were divided into 5 groups (n = 6), namely: healthy control; non-treated diabetic control; and diabetic-rats treated with 200 mg/kg metformin; Labisia pumila ethanol extract (LP) at 150 mg/kg or 300 mg/kg doses. Diabetes was induced by 60 mg streptozotocin /kg intraperitoneal injection. Rats were orally treated daily for ten weeks. Their fasting blood glucose (FBG), neurological functions (hot plate and tail immersion; thermal hyperalgesia; cold allodynia; motor walking function), biomarkers for inflammation and oxidative stress, the neuro-histopathological changes, and brain somatic index were measured.
Results
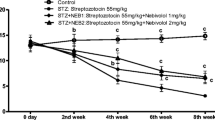
The extract significantly prevented abnormal increases in FBG and decreases in body weight gain. It attenuated behavioral dysfunctions (hot plate and tail immersion; thermal hyperalgesia; cold allodynia; motor walking function), systemic inflammation (serum TNF-α, prostaglandin-E2) oxidative tension (malondialdehyde), histological brain and sciatic nerve injuries in the diabetic-rats, better than Metformin.
Conclusion
LP mitigated neural dysfunction better than metformin partly by amending diabetic systemic inflammation, oxidative tension, and diabetic abnormalities. The nerve injuries were strongly correlated to serum prostaglandin-E2, TNF-α levels, and walking functions. The motor function was correlated to sensory neuronal functions, inflammation, and oxidation. The sensory neuronal functions were more affected by TNF-α than prostaglandin-E2 or oxidation. Diabetic brain and sciatic nerve deteriorations were influenced by serum TNF-α, PGE2, and MDA levels.






Similar content being viewed by others
Data availability
Available on request.
References
Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012;11:521–34. https://doi.org/10.1016/S1474-4422(12)70065-0.
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN. Neuropathic pain, Nat. Rev. Dis. Prim. 3 (2017). https://doi.org/10.1038/nrdp.2017.2.
Serhiyenko VA, Serhiyenko AA. Cardiac autonomic neuropathy: Risk factors, diagnosis and treatment, World. J Diabetes. 2018;9:1–24. https://doi.org/10.4239/wjd.v9.i1.1.
Babizhayev MA. Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: Challenges of dual combination therapy with N-acetylcarnosine lubricant eye d. Fundam Clin Pharmacol. 2012;26:86–117. https://doi.org/10.1111/j.1472-8206.2011.00969.x.
Purwata TE. High TNF-alpha plasma levels and macrophages iNOS and TNF-alpha expression as risk factors for painful diabetic neuropathy. J Pain Res. 2011;4:169–75. https://doi.org/10.2147/JPR.S21751.
Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: Where are we now and where to go? J Diabetes Investig. 2011;2:18–32. https://doi.org/10.1111/j.2040-1124.2010.00070.x.
Chua LS, Lee SY, Abdullah N, Sarmidi MR. Review on Labisia pumila (Kacip Fatimah): Bioactive phytochemicals and skin collagen synthesis promoting herb. Fitoterapia. 2012;83:1322–35. https://doi.org/10.1016/j.fitote.2012.04.002.
Hairi HA, Sofi NSM, Khodari SNK, Jamal JA, Mohamed IN, Shuid AN. Therapeutic Effects of Labisia pumila on Estrogen-deficiency related disorders: An evidence based review. Int J Pharmacol. 2016;12:451–60. https://doi.org/10.3923/ijp.2016.451.460.
Mansor F, Gu HF, Östenson C-G, Mannerås-Holm L, Stener-Victorin E, Nazaimoon W, Mohamud W. Labisia pumila Upregulates Peroxisome Proliferator-Activated Receptor Gamma Expression in Rat Adipose Tissues and 3T3-L1 Adipocytes, Adv. Pharmacol. Sci. 2013 (2013). https://doi.org/10.1155/2013/808914.
Madzuki IN, Lau SF, Che Ahmad Tantowi NA, Mohd Ishak NI, Mohamed S. Labisia Pumila prevented osteoarthritis cartilage degeneration by attenuating joint inflammation and collagen breakdown in postmenopausal rat model. Inflammopharmacology. 26 (2018). https://doi.org/10.1007/S10787-018-0452-6.
Nahar N, Mohamed S, Mustapha NM, Lau SF, Ishak NIM, Umran NS. Metformin attenuated histopathological ocular deteriorations in a streptozotocin-induced hyperglycemic rat model. Naunyn Schmiedebergs Arch Pharmacol. 2020. https://doi.org/10.1007/s00210-020-01989-w.
Dawane JS, Pandit VA, Bhosale MSK, Khatavkar PS. Evaluation of effect of Nishamalaki on STZ and HFHF diet induced diabetic neuropathy in wistar rats, J. Clin. Diagnostic Res. 10 (2016) FF01–FF05. https://doi.org/10.7860/JCDR/2016/21011.8752.
Jain D, Bansal MK, Dalvi R, Upganlawar A, Somani R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J Integr Med. 2014;12:35–41. https://doi.org/10.1016/S2095-4964(14)60001-7.
Kuhad A, Sharma S, Chopra K. Lycopene attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pain. 2008;12:624–32. https://doi.org/10.1016/j.ejpain.2007.10.008.
Hsu M, Stevenson FF. Wallerian degeneration and recovery of motor nerves after multiple focused cold therapies. Muscle and Nerve. 2015;51:268–75. https://doi.org/10.1002/mus.24306.
Altun S, Özdemir S, Arslan H. Histopathological effects, responses of oxidative stress, inflammation, apoptosis biomarkers and alteration of gene expressions related to apoptosis, oxidative stress, and reproductive system in chlorpyrifos-exposed common carp (Cyprinus carpio L.), Environ. Pollut. 2017;230:432–43. https://doi.org/10.1016/J.ENVPOL.2017.06.085.
Decroli E, Manaf A, Syahbuddin S, Syafrita Y, Dillasamola D. The correlation between malondialdehyde and nerve growth factor serum level with diabetic peripheral neuropathy score, Open Access Maced. J Med Sci. 2019;7:103–6. https://doi.org/10.3889/oamjms.2019.029.
Altun S, Özdemir S, Arslan H. Histopathological effects, responses of oxidative stress, inflammation, apoptosis biomarkers and alteration of gene expressions related to apoptosis, oxidative stress, and reproductive system in chlorpyrifos-exposed common carp (Cyprinus carpio L.), Environ. Pollut. 230 (2017) 432–443. https://doi.org/10.1016/j.envpol.2017.06.085.
Im A-R, Kim Y-H, Uddin MR, Chae S, Lee HW, Kim YS, Lee MY. Neuroprotective effects of Lycium chinense miller against rotenone-induced neurotoxicity in PC12 cells. Am J Chin Med. 2013;41:1343–59. https://doi.org/10.1142/S0192415X13500900.
Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: Cellular mechanisms as therapeutic targets, Nat. Rev. Neurol. 2011;7:573–83. https://doi.org/10.1038/nrneurol.2011.137
Coppey LJ, Davidson EP, Rinehart TW, Gellett JS, Oltman CL, Lund DD, Yorek MA. ACE inhibitor or angiotensin II receptor antagonist attenuates diabetic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2006;55:341–8. https://doi.org/10.2337/diabetes.55.02.06.db05-0885.
Laedermann CJ, Abriel H, Decosterd I. Post-translational modifications of voltage-gated sodium channels in chronic pain syndromes, Front. Pharmacol. 6 (2015). https://doi.org/10.3389/fphar.2015.00263.
Kontogiorgis CA, Bompou EM, Ntella M, Vanden Berghe W. Natural products from Mediterranean Diet: From anti-inflammatory agents to dietary epigenetic modulators, Am. J. Community Psychol. 48 (2011) 101–124.
Gholamzadeh S, Zarenezhad M, Montazeri M, Zareikordshooli M, Sadeghi G, Malekpour A, Hoseni S, Bahrani M, Hajatmand R. Statistical analysis of organ morphometric parameters and weights in South Iranian Adult Autopsies, Med. (United States). 96 (2017). https://doi.org/10.1097/MD.0000000000006447.
Wang JQ, Yin J, Song YF, Zhang L, Ren YX, Wang DG, Gao LP, Jing YH. Brain aging and AD-like pathology in streptozotocin-induced diabetic rats, J. Diabetes Res. 2014 (2014). https://doi.org/10.1155/2014/796840.
Niyomchan A, Sricharoenvej S, Lanlua P, Baimai S. Cerebellar synaptopathy in streptozotocin-induced diabetic rats. Int J Morphol. 2019;37:28–35. https://doi.org/10.4067/S0717-95022019000100028.
Østergaard L, Finnerup NB, Terkelsen AJ, Olesen RA, Drasbek KR, Knudsen L, Jespersen SN, Frystyk J, Charles M, Thomsen RW, Christiansen JS, Beck-Nielsen H, Jensen TS, Andersen H. The effects of capillary dysfunction on oxygen and glucose extraction in diabetic neuropathy. Diabetologia. 2015;58:666–77.
Rajchgot T, Thomas SC, Wang JC, Ahmadi M, Balood M, Crosson T, Dias JP, Couture R, Claing A, Talbot S. Neurons and microglia; a sickly-sweet duo in diabetic pain neuropathy, Front. Neurosci. 24 2019/Dr Lau CE Quest. Exam/Curcumin Attenuates Therm. Hyperalgesia a Diabet. Mouse Model of.Pdf. 13 (2019) 1–17. https://doi.org/10.3389/fnins.2019.00025.
Funding
From Universiti Putra Research grant [grant number 9578200].
Author information
Authors and Affiliations
Contributions
Nazmun Nahar (planning, execution, data collection, and manuscript preparation), Seng Fong Lau and Noordin Mohamed Mustapha (attending veterinary clinician for animal studies), and Suhaila Mohamed (grant recipient, main supervisor, project planning coordinator, and manuscript writing/editing, principal researcher). All researchers approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
Nazmun Nahar, Seng Fong Lau, Noordin M. Mustapha, and Suhaila Mohamed have no conflict of interest to declare.
Ethics approval
Institutional Animal Care and Use Committee registration number: (UPM/IACUC/AUP-R095/2017).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nahar, N., Mohamed, S., Mustapha, N.M. et al. Protective effects of Labisia pumila against neuropathy in a diabetic rat model. J Diabetes Metab Disord 21, 1–11 (2022). https://doi.org/10.1007/s40200-021-00905-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-021-00905-0