Abstract
The COVID-19 pandemic requires a rapid understanding of the pathogenesis of the spectrum of the disease and factors associated with varied clinical presentations. Immune dysregulation with a cytokine storm (CS) progressing to ARDS with resemblance to sHLH is suggested as a main cause of tissue injury. Low levels of vitamin D were observed in COVID-19 cases with higher incidence of mortality in 20 European countries, increased risk of severity in COVID-19 contributing to ARDS or fulminant myocarditis and micro vascular thrombosis is proposed. Vitamin D may be protective against acute respiratory tract infections, as it regulates the inflammatory cytokine response of respiratory epithelial cells and macrophages, suppress CS and other manifestations seen in SARS-Cov-2. Hence, it is suggested as one of the therapies in SARS-CoV-2 infection. Major research gaps are identified globally in clinical management and this relationship. There is an imperative requisite to understand the interplay of markers in SARS-CoV-2, its risk factors and potential role of vitamin D to improve clinical outcome by pandemic of COVID-19. We therefore perform this review for understanding the pathophysiology of SARS-CoV-2 infections and the role of vitamin D in combating it.
Similar content being viewed by others
Background
A novel coronavirus was detected in Wuhan, China in December 2019. Patients infected with 2019-nCoV which was later named SARS-Cov-2, were reported with fever, cough, fatigue, and dyspnea. These symptoms were suggestive of viral pneumonia [1] resembling Middle East respiratory syndrome corona-virus (MERS-CoV) and the severe acute respiratory syndrome coronavirus (SARS- CoV) [2]. The number of identified cases rapidly increased and a coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 infection was declared a global pandemic on March 11 by World health Organization (WHO) [3]. Human to human transmission is implicated with droplets and as the main mode of transmission [4]. Worldwide mitigation efforts are ongoing to control the extent of spread of COVID-19.
A wide-ranging spectrum with majority of cases being either asymptomatic or with mild symptoms and few cases presenting as acute respiratory distress syndrome (ARDS) and respiratory failure along with multi-organ dysfunction are seen in COVID-19 [5, 6]. Common presentations include fever, sore throat, cough, headache, generalized weakness, body aches and shortness of breath [5]. The median time for onset of symptoms is reported as 5 days with hospitalization occurring at 7th day and ARDS developing at 8th day [6]. Studies have reported 25–30 % of patients needed intensive care, while overall mortality rate has been reported as 2–3 % [5].
The diagnostics for human coronavirus infections have transformed considerably during this period. Qualitative PCR method using nasopharyngeal or oral swabs or broncho-alveolar lavage fluid is currently the gold standard for diagnosis worldwide. Nasopharyngeal swab may miss diagnosis of SARS-CoV-2 due to poor sample collection technique leading to repetitive sampling to establish a diagnosis [7]. Testing of specific antibodies of SARS-CoV-2 in patient’s blood has also evolved [8]. However, work on vaccine development is ongoing.
Method of literature review
We conducted a literature search on PubMed, Google Scholar and the preprint servers including Medrxiv and SSRN databases using the following MeSH terms: Search 1: (“novel coronavirus” OR “COVID-19” OR “SARS‐CoV‐2” OR “severe acute respiratory syndrome coronavirus 2”) AND (“vitamin D”) Search 2: (“novel coronavirus” OR “COVID‐19” OR “SARS‐CoV‐2” OR “severe acute respiratory syndrome coronavirus 2”) AND (“vitamin D”) AND (“immunity” OR “immune response” OR “innate immunity” OR “Adaptive immunity”); Search 3: : (“novel coronavirus” OR “COVID‐19” OR “SARS‐CoV‐2” OR “severe acute respiratory syndrome coronavirus 2”) AND (“vitamin D”) AND (“Physical barrier”). Search term 4 (“novel coronavirus” OR “COVID‐19” OR “SARS‐CoV‐2” OR “severe acute respiratory syndrome coronavirus 2”) AND (“Pakistan”). Search term 5: (“novel coronavirus” OR “COVID‐19” OR “SARS‐CoV‐2” OR “severe acute respiratory syndrome coronavirus 2”) AND (“vitamin D supplementation”) OR (“vitamin D treatment”). Search term 6: (“vitamin D”) AND (“bacterial infection” OR “viral infection”). The MeSH terms were crosschecked and agreed upon by the co-authors. The articles were short-listed based on the following inclusion criteria: (1) all articles published from January 1, 2020 to November 30, 2020, (2) available as full text in English, (3) categorized as case series, reviews, letters to the editor, original articles or meta-analyses. Titles and abstracts were screened and selected articles were then distributed amongst the co-authors for full text review. Additionally, epidemiological data was obtained from websites of the WHO and other resources all of which have been cited in the following manuscript.
SARS-CoV-2 and Pakistan
In Pakistan greater than 500,000 cases with estimated more than 11000deaths have been reported to date [9]. Pakistan is a LMIC, much populated and the current scenario is not satisfactory due to scarcity of resources. People live in a state of denial and dismissal. Serious actions are needed to curtail the infection in Pakistan to avoid becoming hub for spread of infection worldwide. Threat of an exponential increase in COVID-19 cases is also suspected.
SARS-CoV-2 and body’s immune response
Viral infections induce tissue injury by amplified production of numerous pro-inflammatory cytokines, conscription of pro-inflammatory macrophages and granulocytes, activation of T cells, CD4 and CD8 + T cells. This result in the cytokine storm (CS) also known as macrophage activation syndrome (MAS) or secondary hemophagocytic lymphohistiocytosis (sHLH), which contributes to tissue damage [10]. The antiviral responses offered by host innate and adaptive immunity tries to limit spread of virus, inflammation and clean the infected cells [11, 12]. In this response, a synergistic participation of both innate and adaptive immunity with significant relationship between disease severities, levels of pro-inflammatory cytokines and subsets of immune cells has been demonstrated. Severe COVID-19 shows a CS inexorably progressing to ARDS and resemblance with sHLH [13, 14]. The severity of infection is related to increase in the production of neutrophils, leukocytes, and the neutrophil-lymphocyte ratio (NLR) [15]. Severely affected patients display increased levels of IL-6 as compared to less severe cases. Assessment of pulmonary infiltration in patients with ARDS, identified lung injury (≥ 50 %) is thoroughly interrelated with the augmented level of IL-6 and is considered as a significant cytokine contributing to MAS, due to COVID-19 [16].
Vitamin D and immune response
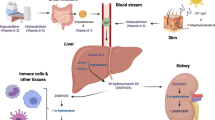
Vitamin D (Cholecalciferol) is formed in the skin when ultraviolet radiation in the sunlight strikes and 7- Dehydrocholesterol (7 -DHC) a derivative of cholesterol in skin, is changed to pre vitamin D, which then isomerizes to vitamin D non-enzymatically. It is then hydroxylated in liver to form 25-hydroxyvitamin D (the main circulating form of Vitamin D). This is further hydroxylated in the kidney to, 1, 25 di-hydroxyvitamin D, (Calcitriol, the metabolically active form of Vitamin D) [17].
The role of Vitamin D as an immune- modulator has been subject of interest among immunologists and dates back to 25 years when it was discovered that monocytes or macrophages from patients with granulomatous diseases like tuberculosis produce calcitriol locally from inactive form of vitamin D [18, 19].The inflammatory cells up-regulate Vitamin D receptors (VDR) and promote conversion of vitamin D metabolites to Calcitriol [20]. Its role in immunity can be put into 3 classes: ‘physical barrier, innate immunity, and adaptive immunity’.
Vitamin D and role as physical barrier
Vitamin D is required to maintain tight junctions, gap junctions, and adherens junctions through E-cadherin [19] among the epithelial cells thereby enhancing the physical barrier which is the first barrier encountered by any pathogen ( bacterial or viral etc.).
Vitamin D & innate immunity
Innate immunity includes the production of both pro-inflammatory and anti-inflammatory cytokines as has been demonstrated with its association in several autoimmune diseases such as systemic lupus erythematosus, Diabetes Mellitus Type I etc. [11, 21]. Vitamin D’s role in innate immunity includes the production of antimicrobial peptides, like cathelicidins (LL-37) (hCAP18) and defensins β2 (DEFB). [19, 22]. Vitamin D affects several of the toll-like receptors, which [23] are activated upon recognition of pathogens, release cytokines and make reactive oxygen species (ROS) and antimicrobial peptides. These peptides, such as cathelicidin, work against the pathogens by disturbing their cell membranes and neutralizing endotoxins and hence help reduce the viral load and its virulence. Cathelicidins are known to have anti-bacterial properties through its in-vitro suppression of not only Mycobacterium tuberculosis. [21, 24] but also includes other bacteria, viruses and fungi. [19] Studies on critically ill patients with severe sepsis found significantly lower concentration of vitamin D associated with low cathelicidins [25].
Vitamin D affects T cell maturation with a skewing away from the inflammatory Th17 phenotype. This way it reduces the production of pro-inflammatory cytokines (IL-17, IL-21) [26] and up regulation of IL 10 mediated responses [24] that cause inflammation which injures the lining of the lungs, eventually causing pneumonia. These cytokines also include tumor necrosis factor α (TNF- α) and interferon γ (INF- γ) which are released by the type 1 T- Helper (Th1) cells.
Vitamin D & adaptive immunity
Vitamin D modulates adaptive immunity by promoting type 2 T helper (Th2) cells to produce cytokines. These cytokines then suppress Th1 cells. Vitamin D promotes induction of the T regulatory cells which help to inhibit inflammatory processes [19, 27]. Cells mediating immune response are known to possess VDR [24].
Vitamin D also plays a role in regulation of immune responses mediated by macrophages and dendritic cells (DC) that are the first line of host defence [28]. Vitamin D modulates the macrophages’ response, preventing them from releasing too many inflammatory cytokine and chemokine such as IL-1, IL-6, IL-8, IL-12 and TNFα by monocytes and increases the expression of anti-inflammatory cytokines [23, 29]. It also augment the phagocytosis of monocytes and induce macrophage autophagy [30] decreasing the CS in COVID-19 infected patients. Therefore antiviral effects of vitamin D include direct interfering with viral replication, and acting as an immune-modulatory and anti-inflammatory agent [31].
Vitamin D & bacterial and viral infections
The increased burden of infectious diseases in regions where vitamin D deficiency is prevalent also suggests an important relationship with host immune response [32]. Vitamin D’s role in acute respiratory tract infections and other viral infections has been studied extensively previously.
The immune-modulatory role of vitamin D in respiratory infections is due to expression of the enzyme 1α-hydroxylase by the airway epithelium, DC and lymphocytes which is essential for the activation of vitamin D within the lungs [18]. The effect of vitamin D in lungs is [33] due to the production of cathelicidin modification of T cell mediated immune responses, reduced innate immune response mediated by DC and inhibition of chemokine. These influence the ability of lung to fight infections as well as respond to allergic stimuli. Ecological studies have suggested a link of vitamin D deficiency with increased predisposition to asthma and respiratory infections including tuberculosis [34].
Vitamin D effects ACE2/Angiotensin (1–7)/MasR axis, enhancing expression of ACE2, which is known to protect against acute lung injury [23] to respiratory tract infections [23] and conditions associated with pneumonia, cytokine hyper production, and ARDS [35]. Prospective studies from UK and USA have shown increase the susceptibility to acute respiratory tract infections due to low serum levels of Vitamin D [23, 36{Monlezun, 2015 #32}].A meta-analysis conducted recently demonstrated a high risk of severe infection and mortality with lower levels of Vitamin D (OR 2.46, 95 % CI 1.65 to 3.66) [37]. Several cross-sectional studies have shown high rates of seasonal influenza associated with low vitamin D levels [38, 39]. Proven efficacy of vitamin D has been established in seasonal influenza in the northern hemisphere where winters are harsh, and residents have low vitamin D levels [40]. It is due to these mechanisms and a recent study that vitamin D was suggested as a novel drug for influenza A H5N1 virus-induced lung injury [5]. Not surprisingly, results of therapeutic administration of Vitamin D to patients with Influenza using nasopharyngeal swab as an objective measure showed a 42 % reduction in the risk of infection [22]. A study done on animal models showed that vitamin D reduced lung permeability due to its modulatory effect on the RAS and the expression of ACE2, and therefore vitamin D pretreatment proved to be useful in animal models of ARDS [41]. Also, certain VDR gene alleles have been found to be related with amplified vulnerability to respiratory infections [42]. Moreover, vitamin D insufficiency has been linked with increased incidence of asthma exacerbations due to triggering by viral infections in both children [43] and adults [44].
Amongst other viral infections, Vitamin D’s effectiveness as an adjuvant therapy alongside other antiretroviral agents has also been suggested in Dengue Virus [45] and HIV infection [46]. Vitamin D supplementation has also been seen to increase the CD4 + T cell count in HIV infection [27].
Vitamin D and role of supplementation
Vitamin D supplementation are thought to reduce risks of infection by improving the expression of glutathione reductase and glutamate–cysteine ligase modifier subunit, which are related to antioxidation. Further, increased production of glutathione makes ascorbic acid (vitamin C) available which has antimicrobial activities. Amongst other micronutrients vitamin C and Zinc supplementations are also proven to help boost immunity [19]. For the same reasons, vitamin D supplementation has reportedly been of benefit in reducing severity of viral illness and inducing early recovery by several studies and associated with good overall outcomes [30].
However, the recommended dosage for vitamin D supplementation are not clear and so are the optimal levels. Recommended daily allowance in most guidelines is 600–4000 IU/d and a serum concentration of above 20 ng/mL is considered as sufficient. Studies have proposed higher levels for high-risk groups against virus infection and different dosing regimens has been proposed. However, with higher doses hypervitaminosis and toxicity has been reported recently; questioning the safety of high serum vitamin D levels [47]. Regular oral vitamin D intake (in doses up to 2000 IU/d without additional bolus) was declared to be safe and protective against ARDS, particularly in subjects with vitamin D deficiency [48].
Vitamin D and SARS-CoV-2 infection
Higher levels of ACE2 have been associated with better COVID health outcomes in previous studies [23]. Vitamin D enhances the expression of ACE2 [23, 41]. It has also been observed to decrease acute lung injury in mice by modulating effects on renin-angiotensin system (RAS) and ACE2/Angiotensin signaling pathway [48].
Vascular injuries are associated with COVID-19 whereas vitamin D increases vascular endothelial growth factor (VEGF) production which promotes vascular endothelial repair [47]. Vitamin D scarcity has also been labeled to cause increased risk for thrombosis, endothelial dysfunction and pathological changes to the vascular system [27, 47].
Since vitamin D insufficiency (VDI) promotes RAS, which can lead to chronic cardiovascular disease as well as impaired lung function [27]. Therefore, a possible role of vitamin D in SARS-CoV-2 infection on the basis of its impact on innate and adaptive immunity, effects on the cardiovascular system, derangement of the immune response and its prothrombotic effects is been considered. Interest in exploring therapeutic potential of vitamin D in COVID-19, akin to IL-6 inhibition with Tocilizumab is also gaining ground [49] considering low cost, easy availability, and comparatively good safety profile.
Other evidence that supports the possible role of vitamin D in COVID-19 is on the basis of outbreak occurring in winter, less number of cases in the Southern Hemisphere (deaths and hospitalizations) in Northern latitudes [48], high mortality rate in these areas [48], ARDS with increase in fatality rates and chronic disease comorbidity, with lower vitamin D concentration [19]. The elderly, also happen to be the one with low vitamin D levels [23]. This is because with increasing age vitamin D levels fall, mainly due to decreased exposure to sunlight and cutaneous synthesis which is the main contributor of vitamin D in the body [50, 23]. Other sources of the vitamin include fish and dairy products. These are found in high quantity in Scandinavian diets. And this is why if we look at Europe for example, the Scandinavian countries (Norway and Denmark) have VDI rates of 15–30 % and COVID-19 is much less severe here as opposed to Italy, Greece and Spain where VDI rates are around 70–90 %, and the disease has been severe [50]. Countries with high number of COVID-19 cases such as Italy and Switzerland have mean circulating vitamin D levels of below 30 nmol/L and 23 nmol/L respectively [50, 48].
Studies have also been conducted in in-patient setting order to see the prevalence of VDI with COVID-19 [50]. In an observational study from three South Asian Countries in 212 patients [51], multinominal logistic regression to analyze influence of vitamin D deficiency on COVID-19 outcomes was conducted. The study monitored vitamin D levels from the onset of symptoms every seven days. Vitamin D deficiency was associated with an increase in adverse clinical outcomes in patients severely infected with SARS-CoV-2 infection, compared to those with mild illness [51]. This important observation awaits peer review for ascertainment, however, this finding is important for clinicians and policy makers alike, as to date there is no standard of care or definitive vaccine to protect against SARS-CoV-2 infection. In this region most are LMIC, supplementation of vitamin D can be considered to prevent complications of COVID-19 as heath care resources are limited and not accessible to all.
In a recently published intensive review [52] on implications of vitamin D in prevention and treatment of COVID-19, higher morbidity and mortality in the African-American population in the US correlated with low vitamin D status explaining a positive causal relation with poor outcomes [52]. Authors propose increasing serum and tissue concentrations of vitamin D in the acute phase of severe infections to prevent lung complications [52]. The author also emphasized that while optimal vitamin D levels enhanced epithelial expression of Th 1 cells, low level of vitamin D causes release of INF-γ culminating in CS in the advanced stage of SARS-CoV-2 infection [53]. A population based survey from John Hopkins University also demonstrated COVID-19 infection rate three times higher as well as a six times higher mortality rate in African-Americans compared with Caucasians [54].
From Southeast Asia, a study on 780 patients infected with COVID-19 in Indonesia [55], reported significant co-morbidity in 80 % of patients with vitamin D deficiency. In this pre-print yet significant study, mortality was significantly higher with insufficient vitamin D status, (OR = 7.63; p < 0.001). Similarly, in vitamin D deficient cases, the odds of mortality were 10.12 greater compared to patient with normal concentration, (OR = 10.12; p < 0.001) [55]. Nevertheless, with emerging evidence from observational data, there is heightened need for experimental studies and intervention trials to understand the role of vitamin D in COVID-19 outcomes.
A large observational population study from Israel [56] compared risk of COVID-19 infection with prevalence of Vitamin D deficiency in 200 localities. In this largest study to date on impact of vitamin D on COVID 19, an additional 52,405 patients were included and matched with 524,050 controls to examine the effect of baseline Vitamin D level, supplementation in preceding months and COVID-19 incidence [56]. The researchers found a significant correlation between risk of acquisition of Covd-19 with underlying low Vitamin D and risk was highest with severe deficiency [56].
As far as disease severity of COVID − 19 is concerned, Vitamin D has shown to modulate disease course in a case-control study on Iranian patients [57]. Low Vitamin D not only contributed to mortality along with increased neutrophil to lymphocyte ratio (NLR) but were associated with higher angiotensin converting enzyme (ACE) level in deceased patients [57]. The patients who died had profoundly lower mean vitamin D concentrations compared to patients without COVID-19. Consistent with previous studies, increased ACE level was a marker of poor outcomes in COVID 19 in this study [57].
Hypoxemic respiratory failure is a life-threatening complication of COVID 19 disease [58]. A study on implication of Vitamin D on survival among hospitalized critically ill patients showed a higher probability of mortality in patients with severe Vitamin D deficiency [58]. A value of less than 10ng/dl was shown to be associated with 5 % risk of death in severe COVID 19 disease [58]. Vitamin D insufficiency has also been found in regulating immune response to fatal cytokine release syndrome (CRS) associated with severe symptomatic COVID 19 and mortality in affected patients in causal inferential and predictive models [59].
In a randomized open label clinical trial [60] conducted at an Intensive care unit of a university teaching hospital in Spain, patients were administered calcifediol through electronic randomization and others were not given Vitamin D [60]. Only one patient in the calcifediol group had to be transferred to ICU and this was an outcome measure whereas, 13 out of 26 patients who were not randomized to calcifediol required ICU [60].
Vitamin D deficiency plays a role in prothrombotic state responsible for coagulopathy and thrombotic complications like pulmonary embolism, stroke and deep vein thrombosis [61]. Poor outcomes in COVID 19 are also characterized by coagulopathy [62]. In the United Kingdom, Vitamin D deficiency was found to be higher in ethnic minorities and contributed to increased incidence of thrombotic events and mortality in this population [63]. It also leads to clinical disease severity by down regulating T-regs (T regulatory lymphocytes) which are found to be low in patients with Vitamin D deficiency [63]. Comorbidities like Diabetes Mellitus and Hypertension are also found to be associated with worse outcomes in COVID 19 due to the high inflammatory response which can be ameliorated by Vitamin D3 supplementation [63].
Recommendations & way forward
In summary, the role of vitamin D in modifying and reducing the inflammatory cytokine response of respiratory epithelial cells and macrophages to various pathogens has been documented [64]. Moreover, it is expected that vitamin D might protect against SARS-CoV-2 infection by avoiding the CS and the subsequent ARDS contributing to increased mortality [65]. The antiviral effects of vitamin D, which can directly reduce viral replication may be a supportive factor on immunomodulation and anti-inflammation [31]. These effects could be used in helpful in combating the immune evasion mechanisms caused by SARS-CoV-2 [66]. In the same context, the protective role of vitamin D has been testified in pneumonia, cytokine hyperproduction, and ARDS [67, 68]. Hence, Vitamin D can serve as a potential immune-modulator in COVID-19 infection due to its immune regulating function being supported by considerable clinical evidence [49, 69]. Although not prospectively validated in a randomized control trial; the role of vitamin D in attenuating hyper-acute inflammatory response in severe COVID-19 can be determined by studying impact of vitamin D deficiency on markers like CRP and IL-6 [59]. While centers worldwide routinely utilize CRP levels to assess CS, levels of vitamin D before development of CS and afterwards can predict reliability of vitamin D as an immune-modulating agent that can alter IL-6 activity and serve as a therapeutic target in treatment of CS [59]. Vitamin D3 supplementation is inexpensive and readily available. Therefore, on the current covid-19 pandemic where a cause-and-effect relationship continues to be explored, it is prudent to follow public health guidelines to ensure vitamin D3 adequacy and daily supplementation with 1000–2000 IU per day.
Despite all of this, there are knowledge gaps and demonstration of a cause-effect relationship with vitamin D intervention has not been established so far. Large, multi-center, placebo-controlled clinical trials in patients with varying severity of COVID-19 infection with vitamin D are recommended. Furthermore, excess vitamin D supplementation could result in hypervitaminosis and toxicity and hence caution should be exercised in replacing vitamin D with clinical correlation. Equal emphasis should be given to fortified food sources and lifestyle. This will help in improving the immune system and will provide lasting health benefits while evidence is beingestablished for the specific use of vitamin D in COVID-19 patients.
Data availability
All data presented in this study is included in this published article.
References
Adhikari SP, Meng S, Wu Y-J, Mao Y-P, Ye R-X, Wang Q-Z, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):1–12.
Jiang F, Deng L, Zhang L, Cai Y, Cheung C, Xia Z. COVID-19. J Gen Intern Med. 2019;2020:1–5.
Organization WH. Coronavirus disease (COVID-19) pandemic 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 27 Jan 21.
Kong I, Park Y, Woo Y, Lee J, Cha J, Choi J, et al. Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res Perspect. 2020;11(1):8–14.
Huang F, Zhang C, Liu Q, Zhao Y, Zhang Y, Qin Y, et al. Identification of amitriptyline HCl, flavin adenine dinucleotide, azacitidine and calcitriol as repurposing drugs for influenza A H5N1 virus-induced lung injury. PLoS Pathog. 2020;16(3):e1008341.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
Loeffelholz MJ, Tang Y-W. Laboratory diagnosis of emerging human coronavirus infections–the state of the art. Emerg Microbes Infect. 2020;9(1):747–56.
Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92(9):1518–24.
Worldometer. Coronavirus Cases. Pakistan 2021. Available from: https://www.worldometers.info/coronavirus/country/pakistan. Accessed 27 Jan 21.
McGonagle D, Sharif K, O’Regan A, Bridgewood C. Interleukin-6 use in COVID-19 pneumonia related macrophage activation syndrome. Autoimmun Rev. 2020;19:102537.
Li CK-f, Wu H, Yan H, Ma S, Wang L, Zhang M, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181(8):5490–500.
Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827.
Guo Y-R, Cao Q-D, Hong Z-S, Tan Y-Y, Chen S-D, Jin H-J, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res. 2020;7(1):1–10.
Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system.Cytokine Growth Factor Rev. 2020;53:25-32.
Yang A-P, Liu J, Tao W, Li H-M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients.Int Immunopharmacol 2020;84:106504.
Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, et al. Elevated levels of interleukin-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1):128-136.e4.
Maha Q, Masood L, Rehman R. Vitamin D receptor polymorphism and male factor infertility. J Pak Med Assoc. 2019;69(4):603–4.
Hansdottir S, Monick MM. Vitamin D effects on lung immunity and respiratory diseases. Vitam Horm. 2011;86:217–37.
Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988.
Lee J, Van Hecke O, Roberts N. Vitamin D: A rapid review of the evidence for treatment or prevention in COVID-19. Centre for Evidence-Based Medicine Website. 2020. Available from: https://www.cebm.net/covid-19/vitamin-d-a-rapid-review-of-the-evidence-for-treatment-or-prevention-in-covid-19/. Accessed 12 Dec 20.
Kongsbak M, von Essen MR, Levring TB, Schjerling P, Woetmann A, Ødum N, et al. Vitamin D-binding protein controls T cell responses to vitamin D. BMC Immunol. 2014;15(1):35.
Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5(7):2502–21.
Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32(7):1195–8.
Lang P, Samaras N, Samaras D, Aspinall R. How important is vitamin D in preventing infections? Osteoporos Int. 2013;24(5):1537–53.
Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA Jr, Koeffler HP, et al. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis. 2009;48(4):418–24.
Andress D. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69(1):33–43.
Tian Y, Rong L. Covid-19 and vitamin D-authors’ reply. Aliment Pharmacol Ther. 2020;51(10):995–6.
Bruce D, Ooi JH, Yu S, Cantorna MT. Vitamin D and host resistance to infection? Putting the cart in front of the horse. Exp Biol Med. 2010;235(8):921–7.
Aranow CJ. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–6.
Konijeti GG, Arora P, Boylan MR, Song Y, Huang S, Harrell F, et al. Vitamin D supplementation modulates T cell–mediated immunity in humans: results from a randomized control trial. J Clin Endocrinol Metab. 2016;101(2):533–8.
Teymoori-Rad M, Shokri F, Salimi V, Marashi SM. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29(2):e2032.
Bischoff-Ferrari HA, Dawson-Hughes B, Platz A, Orav EJ, Stähelin HB, Willett WC, et al. Effect of high-dosage cholecalciferol and extended physiotherapy on complications after hip fracture: a randomized controlled trial. Arch Intern Med. 2010;170(9):813–20.
Amento EP. Vitamin D and the immune system. Steroids. 1987;49(1–3):55–72.
Coussens AK. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem Photobiol Sci. 2017;16(3):314–38.
Jakovac H. COVID-19 and vitamin D—Is there a link and an opportunity for intervention? Am J Physiol-Endocrinol Metab. 2020;318(5):E589-E.
Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5(6):e11088.
Pham H, Rahman A, Majidi A, Waterhouse M, Neale RE. Acute respiratory tract infection and 25-hydroxyvitamin D concentration: a systematic review and meta-analysis. Int J Environ Res Public Health. 2019;16(17):3020.
Cannell J, Vieth R, Umhau J, Holick M, Grant W. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–40.
Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019;23(2):1–44.
Goncalves-Mendes N, Talvas J, Dualé C, Guttmann A, Corbin V, Marceau G, et al. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front Immunol. 2019;10:65.
Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide–induced acute lung injury via regulation of the renin–angiotensin system. Mol Med Rep. 2017;16(5):7432–8.
Jolliffe DA, Greiller CL, Mein CA, Hoti M, Bakhsoliani E, Telcian AG, et al. Vitamin D receptor genotype influences risk of upper respiratory infection. Br J Nutr. 2018;120(8):891–900.
Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126(1):52–8 e5.
Confino-Cohen R, Brufman I, Goldberg A, Feldman B. Vitamin D, asthma prevalence and asthma exacerbations: a large adult population‐based study. Allergy. 2014;69(12):1673–80.
Martínez-Moreno J, Hernandez JC, Urcuqui-Inchima S. Effect of high doses of vitamin D supplementation on dengue virus replication, Toll-like receptor expression, and cytokine profiles on dendritic cells. Mol Cell Biochem. 2020;464(1–2):169–80.
Jiménez-Sousa M, Martínez I, Medrano LM, Fernández-Rodríguez A, Resino S. Vitamin D in human immunodeficiency virus infection: influence on immunity and disease. Front Immunol. 2018;9:458.
Tian Y, Rong L. Does vitamin D have a potential role against COVID-19? Authors’ reply.Aliment Pharmacol Ther. 2020;52(2):410-411.
Panarese A, Shahini E. Letter: Covid-19, and vitamin D. Aliment Pharmacol Ther. 2020;51(10):993–5.
Sharma M, Panda NK. Proteomic profiling of protease-primed virus-permissive Caco-2 Cells Display Abortive-Interferon Pathway and Deregulated thromboinflammatory SERPINS. Preprints. 2020. https://doi.org/10.20944/preprints202006.0206.v1.
Lau FH, Majumder R, Torabi R, Saeg F, Hoffman R, Cirillo JD, et al. Vitamin D insufficiency is prevalent in severe COVID-19. medRxiv. 2020. https://doi.org/10.1101/2020.04.24.20075838.
Alipio M, Vitamin D. Supplementation Could Possibly Improve Clinical Outcomes of Patients Infected with Coronavirus-2019 (COVID-19). SSRN Electron J. 2019;2020:1–9.
Mansur JL, Tajer C, Mariani J, Inserra F, Ferder L, Manucha W. El suplemento con altas dosis de vitamina D podría representar una alternativa promisoria para prevenir o tratar la infección por COVID-19. Clín Investig Arterioscler. 2020;32(6):267–77.
Theron M, Huang K-J, Chen Y-W, Liu C-C, Lei H-Y. A probable role for IFN-γ in the development of a lung immunopathology in SARS. Cytokine. 2005;32(1):30–8.
Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891–2.
Raharusun P, Priambada S, Budiarti C, Agung E, Budi C. Patterns of COVID-19 mortality and vitamin D: an Indonesian study. SSRN Electron J. 2020;7:1–12. https://doi.org/10.2139/ssrn.3585561.
Israel A, Cicurel AA, Feldhamer I, Dror Y, Giveon SM, Gillis D, et al. The link between vitamin D deficiency and Covid-19 in a large population. medRxiv. 2020. https://doi.org/10.1101/2020.09.04.20188268.
Mardani R, Alamdary A, Mousavi Nasab SD, Gholami R, Ahmadi N, Gholami A. Association of vitamin D with the modulation of the disease severity in COVID-19. Virus Res. 2020;289:198148.
Carpagnano GE, Di Lecce V, Quaranta VN, Zito A, Buonamico E, Capozza E, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest. 2021;44(4):765–71.
Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. medRxiv. 2020;2020.04.08.20058578.
Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751.
Mohammad S, Mishra A, Ashraf MZ. Emerging role of vitamin D and its associated molecules in pathways related to pathogenesis of thrombosis. Biomolecules. 2019;9(11):649.
Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362.
Weir EK, Thenappan T, Bhargava M, Chen Y. Does vitamin D deficiency increase the severity of COVID-19? Clin Med (Lond, Engl). 2020;20(4):e107–e108.
Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. J Nutrients. 2015;7(6):4240–70.
Panarese A, Pesce F, Porcelli P, Riezzo G, Iacovazzi PA, Leone CM, et al. Chronic functional constipation is strongly linked to vitamin D deficiency. World J Gastroenterol. 2019;25(14):1729.
Guo Y-R, Cao Q-D, Hong Z-S, Tan Y-Y, Chen S-D, Jin H-J, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res. 2020;7(1):1–10.
Hong M, Xiong T, Huang J, Wu Y, Lin L, Zhang Z, et al. Association of vitamin D supplementation with respiratory tract infection in infants. Matern Child Nutr. 2020;16:e12987.
Zhou Y-F, Luo B-A, Qin L-LJM. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine (Baltimore). 2019;98(38):e17252.
Li Q, Dai Z, Cao Y, Wang L. Association of C-reactive protein and vitamin D deficiency with cardiovascular disease: A nationwide cross‐sectional study from National Health and Nutrition Examination Survey 2007 to 2008. Clin Cardiol. 2019;42(7):663–9.
Author information
Authors and Affiliations
Contributions
AH and RR conceived idea, contributed to literature search and writing the manuscript.
NN, NNS and QM did literature search and contributed in writing of manuscript.
All authors contributed equally to the manuscript.
All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, A.H., Nasir, N., Nasir, N. et al. Vitamin D and COVID-19: is there a role?. J Diabetes Metab Disord 20, 931–938 (2021). https://doi.org/10.1007/s40200-021-00775-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-021-00775-6