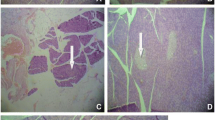
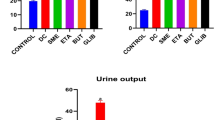
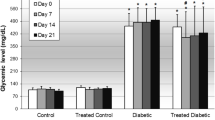
Abstract
Purpose
The current study aimed to determine the antidiabetic effects of leaf extract of Ficus asperifolia in streptozotocin-induced diabetic rats.
Methods
A total of ninety-five (95) adult rats were used for the experiment. The whole study protocol was divided into three sets of individual experiments. The animals were divided randomly into seven groups of five rats each. The rats were given a diet supplemented with 50 g high fat to 50 g vital feeds for two weeks. The study lasted 28 days with daily administration and weekly blood glucose and body weight check. At the end of the experiment protocol, the rats were fasted overnight and were anesthetized. Blood was collected via cardiac puncture from each animal for biochemical analysis. Metglim 3.38 mg/kg bodyweight was used as positive control. Diabetes was induced using streptozotocin (35 mg/kg in 0.1 M phosphate-buffered saline) intraperitoneally for 5 days. Phytochemicals were analyzed in both extract and fractions.
Results
Phytochemical screening revealed the presence of alkaloids, saponins, flavonoids, glycosides, tannins, carotenoids, terpenes, and steroids in both extract and fractions. Proteins, carbohydrates, and fats were detected by systematic molecular analysis. Fraction 1 of plant extracts prevented glucose-induced hyperglycaemia 30 min’ post glucose load in all rats. Streptozotocin treatment caused a significant increase (p˂0.05) in blood sugar, total cholesterol, triacylglycerol, low-density lipoproteins and a significant (p˂0.05) decrease in food intake, body weight and high-density lipoproteins in diabetic rats.
Conclusion
Treatment with the extract significantly improved the derangements caused by streptozotocin. These results suggest that the leaf extracts of Ficus asperifolia could serve as a potential treatment for diabetes therapy.



Similar content being viewed by others
Change history
13 May 2020
The author names are incorrectly displayed in the original article and should be Faith Pwaniyibo Samson (F.P. Samson), Ambrose Teru Patrick (A.T. Patrick), Margret Samuel Nadro (M.S. Nadro), and Jin Jahng Wan (J.J. Wan).
References
Arbonnier M. Trees, shrubs and lianas of west African dry zones, vol. 3. 1stEdition ed: CIRAD Margraf Publishers GMBH MNHN, USA; 2004. p. 574.
J. B Harborne, (1984). Phytochemical Methods-A guide to Modern Techniques of Plant Analysis 2nd Edition New York Chapman and Hall 5. (10).
Sofowora GG, Kohli U, Muszkat M, Friedman EA, Scheinin M, Wood AJ, et al. ADRA2C deletion 322–325- effects on pain perception, before and after Dexmedetomidine. Circ Cardiovasc Genet. 2008;4:179–87. https://doi.org/10.1161/CIRCGENETICS.110.957662.
Evans WC. Trease and Evans. Pharmacognosy. 15th ed. Edinburgh: W.B Saunders; 2002.
AOAC (2000). Association of Official Analytical Chemists. (18thed.) Official methods of analysis. Washington D.C 18-62.
Noureddini H, Byun J. Dilute-acid pre-treatment of distillers’ grains and corn fiber. Bioresour Technol. 2010;101:1060–7. https://doi.org/10.1016/j.biortech.2009.08.094.
Latimer GW. Official methods of analysis of AOAC international. AOAC international. Gaithersburg, MD, USA; 2016.
Kjeldahl JN. Method for determination of nitrogen in organic bodies. Fresenius J Anal Chem. 1883;22:366–82. https://doi.org/10.1007/BF01338151.
Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacology Research. 2005;52:313–20. https://doi.org/10.1016/j.phrs.2005.05.004.
Aja PM, Ibiam UA, Uraku AJ, Orji OU, Offor CE, Nwali BU. Comparative proximate and mineral composition of Moringa oleifera leaf and seeds. Global Advanced Research Journal of Agricultural Science. 2013;2:137–41.
Kriushnapriya S, Dhinagar K, Malathy S, Mani K. Database for vegetable phytochemicals and their mechanism of action. Bioinformation. 2012;8:492–5. https://doi.org/10.6026/97320630008492.
Omoniwa BP, Luka CD. Antidiabetic and toxicity evaluation of aqueous stem extract of Ficus asperifolia in Normal and Alloxan-induced diabetic albino rats. Asian Journal of experimental biological sciences. 2012;3:726–32.
Patrick AT, Samson FP, Jalo K, Thagriki D, Umaru HA, Madusolumuo MA. Invitro antioxidant activity and phytochemicals evaluation of aqueous and Methanolic stem bark extracts of Pterocarpus erinaceus. World Journal of Pharmaceutical Research. 2016;5:134–51.
S. Y. Pan, S. F. Zhou, S. H. Gao, Z. L. Yu, S. F. Zhang, M. K. Tang et al. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evidence-Based Complementary and Alternative Medicine, Article ID: (2013) 627375. doi: https://doi.org/10.1155/2013/627375.
Jung M, Park M, Lee HC, Kang YH, Kang ES, Kim SK. Anti-diabetic agents from medicinal plants. Current Medical Chemistry. 2006;13:1203–8.
Griffiths RC, Watson AA, Kizu H, Asano N, Sharp HJ, Jones MG, et al. The isolation from Nicandra physalodes and identification of the 3-O-(−d glucopyranoside of 1a, 2b, 3a, 6a-tetrahydroxy-nor-tropane (calystegineB1). Tetrahedron Letter. 1996;37:3207–8.
Lacaille-Dubois MA, Wagner H. Bioactive saponins from plants: an update. Stud Nat Prod Chem. 2000;21:633–87.
Saxena M, Saxena J, Nema R, Singh D, Gupta A. Phytochemistry of medicinal plants. Journal of Pharmacognosy and Phytochemistry. 2013;1:168–82.
Abdel-Zaher AO, Salim SY, Assaf MH, Abdel-Hady RH. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J Ethnopharmacol. 2005;101:129–38. https://doi.org/10.1016/j.jep.2005.04.007.
Smith AYR, Adanlawa IG. Hypoglycaemic effects of Saponin from the root of Garcinia kola (bitter kola) on Alloxan-induced diabetic rats. Journal of Drug Delivery and Therapeutics. 2012;2:9–12. https://doi.org/10.22270/jddt.v2i6.338.
S. Salvamani, B. Gunasekaran, N. Shaharuddin, S. A. Ahmad and M. Y. Shukor. Antiatherosclerotic effects of plants of flavonoids. Biomed Res Int. (2014) 1–11, Article I.D: 480258. doi: https://doi.org/10.1155/2014/480258.
Johnston K, Sharp P, Clifford M, Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Letter. 2005;579:1653–7. https://doi.org/10.1016/j.febslet.2004.12.099.
Roberto T, Bonfigli AR, Stefano G, Valeria DN, Antonio C. The possible role of flavonoids in the prevention of diabetic complications. Nutrients. 2016;8:310. https://doi.org/10.3390/nu8050310.
Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. Journal of Diabetes Metabolic Disorders. 2013;12:43. https://doi.org/10.1186/2251-6581-12-43.
Kumar K. Roles of tannins on animal’s performance: an overview. Journal of Natural Remedies. 2011;11:82–9.
R. Kumari, D. K. Patel, S. K. Prasad, D. Laloo, S. Krishnamurthy, S. Hemalatha. Type 2 antidiabetic activity of bergenin from the roots of Caesalpinia digyna Rottler.Fitoterpia, 83 (2012) 395-401. DOI: https://doi.org/10.1016/j.fitote.2011.12.008.
Omoniwa BP, Luka CD, Soji-Omoniwa O. Effect of aqueous leaf extract of Ficus asperifolia on cardiac enzymes and lipid profile in male albino rats. J Med Sci. 2013. 2013;13:373–8. https://doi.org/10.3923/jms.2013.373.378.
Hayat IA, Ahmad A, Ahmed S, Khalil S, Gulfraz M. Exploring the potential of red kidney beans (Phaseolus vulgaris L.) to develop protein-based product for food applications. Journal of Animal and Plant Science. 2014;24:860–8.
Weickert MO, Mohlig M, Koebnick C, Holst JJ, Namsolleck P, Ristow M, et al. Impact of cereal fibre on glucose–regulating factors. Diabetologia. 2005;48:2343–53. https://doi.org/10.1007/s00125-005-1941-x.
Post RE, Mainous AG, King DE, Simpson KN. Dietary fiber for the treatment of type 2 diabetes mellitus. A meta-analysis Journal of American Board of Fam Med. 2012;25:16–23. https://doi.org/10.3122/jabfm.2012.01.110148.
Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. Journal of Education and Health Promotion. 2014;3:1. https://doi.org/10.4103/2277-9531.127541.
Grover JK, Yadav S, Vats V. Medicinal plants of India with antidiabetic potential. J Ethnopharmacol, 81. 2002:81, 100. https://doi.org/10.1016/s0378-8741(02)00059-4.
Anitha M, Sakthidevi G, Muthukumarasamy S, Mohan VR. Effect of Cynoglossum zeylanicum (Vehl ex Hornem) Thunb. Ex Lehm on Oral glucose tolerance in rats. J App Pharm Sci. 2012;2:075–8. https://doi.org/10.7324/JAPS.2012.21113.
Nadro MS, Elkanah G. Hypoglycaemic effect of fractions and crude Methanolic leaf extract of Phyllantus fraternus in streptozotocin-induced diabetic and normal rats. Journal of Medicinal Plants Research. 2017;11:58–65. https://doi.org/10.5897/JMPR2016.6300.
Mohammad SA, Nabi SA, Marella S, Thandaiah KT, Kumar MVJ, Rao CA. Phytochemical screening and antihyperglycemic activity of Heliotropium indicum whole plant in Streptozotocin induced diabetic rats. Journal of Applied Pharmaceutical Science. 2014;4:065–71. https://doi.org/10.7324/JAPS.2014.41212ISSN2231-3354.
Gupta N, Jain UK, Pathak AK. Wound healing properties of Artocarpus heterophyllus lam. Ancient Science Life. 2009;28:36–7.
Tapas A, Sakarkar D, Kakde R. Flavonoids as nutraceuticals: a review. Tropical Journal of Pharmacology Research. 2008;7:1089–99.
Nadro MS, Samson FP. The effects of Balanite aegyptiaca kernel cake as supplement on Alloxan-induced diabetes mellitus in rats. Journal of Applied Pharmaceutical Science. 2014;4:58–61. https://doi.org/10.7324/JAPS.2014.40111.
Patrick AT, Samson FP, Umaru HA. The effects of crude extract of ginger (Zingiber officinale) on some lipid profile parameters in high fat fed rats. Journal of Biology, Agriculture and Healthcare. 2015;5:123–7.
Yadav JP, Saini S, Kalia AN, Dangi AS. Hypoglycaemic and hypolipidaemic activity of ethanolic extract of Salvadora oleoides in normal and alloxan-induced diabetic rats. Indian Journal of Pharmacology. 2008;40:23–7. https://doi.org/10.4103/0253-7613.40485.
Yusufoglu HS, Soliman GA, Abdel-Rahman RF, Abdel-Kader MS, Ganaie MA, et al. Antihyperglycemic and antihyperlipidemic effects of Ferula duranii in experimental type 2 diabetic rats. Int J Pharmacol. 2015;11:532–41. https://doi.org/10.3923/ijp.2015.532.541.
Ogunyinka BI, Oyinloye BE, Osunsanmi FO, Kappo AP, Opoku AR. Comparative study on proximate, functional, mineral, and anti-nutrient composition of fermented, defatted, and protein isolate of Parkia biglobosa seed. Journal of Food Science and Nutrition. 2017;5:139–47. https://doi.org/10.1002/fsn3.373.
Abdel-Hassan IA, Abdel-Barry JA, Mohammeda ST. The hypoglycaemic and antihyperglycemic effect of Citrullus colocynthis fruit aqueous extract in normal and Alloxan diabetic rabbits. J Ethnopharmacol. 2000;71:325–30. https://doi.org/10.1016/s0378-8741(99)00215-9.
Jung UJ, Lee MK, Jeong KS, Choi MS. The hypoglycaemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J Nutr. 2004;134:2499–2503. https://doi.org/10.1093/jn/134.10.2499.
Acknowledgments
The authors thank the HOD Department of Biochemistry Professor S. Sarkiyayi for making available the necessary facilities for the conduct of this research and we also appreciate Department technicians for their technical assistance and Professor M. A. Madusolumuo for editorial guidance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Pwaniyibo, S.F., Teru, P.A., Samuel, N.M. et al. Anti-diabetic effects of Ficus Asperifolia in Streptozotocin-induced diabetic rats. J Diabetes Metab Disord 19, 605–616 (2020). https://doi.org/10.1007/s40200-020-00524-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00524-1