Abstract
Background
Peripheral neuropathy is a dose-limiting adverse effect of vincristine (VCR) in cancer chemotherapies. Dapsone is commonly used for the prevention of opportunistic infections following cancer therapies. Therefore, a high rate of VCR and dapsone co-administration has occurred in leukemias. Recently neuroprotective effects of dapsone have been reported in various diseases.
Objectives
Regarding the physiopathology of VCR-induced peripheral neuropathy (VIPN) and dapsone neuroprotection, this study evaluated the effect of dapsone on VIPN.
Methods
VIPN was induced by VCR injection (0.5 mg/kg IP, every other day, 1 week) in male Wistar rats. In the treatment group, dapsone(12.5 mg/kg IP, 1 week) was injected 30 min before VCR. Hot plate, Von Frey, motor neuron conduction velocity (MNCV), and histopathological tests were applied. The levels of TNF-α and NF-kB in the sciatic nerve and caspase-3 activity in dorsal root ganglion were measured by the ELISA method. The levels of malondialdehyde (MDA) and Glutathione (GSH) in the sciatic nerve were measured by spectrophotometry and colorimetric assays.
Results
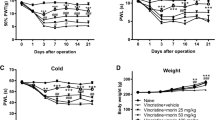
VIPN was observed as araised thermal and mechanical threshold, reduced MNCV, and sciatic nerve demyelination. However, dapsone reduced the mechanical and thermal threshold and improved the MNCV. Also, dapsone reduced TNF-α, NF-kB, MDA, and Caspase-3 activity, and increased the GSH level in the sciatic nerve. Moreover, dapsone prevented VCR-induced demyelination in the sciatic nerve.
Conclusion
This research demonstrated that dapsone could be used as a protective drug against VIPN. It improves the impaired thermal and mechanical sensations by reducing inflammatory, oxidant, and apoptosis factors and preventing demyelination in the sciatic nerve.
Graphical abstract







Similar content being viewed by others
Data availability
All data details will be available upon request.
References
Mora E, et al. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res. 2016;6(11):2416.
Williams S, et al. Methemoglobinemia in children with acute lymphoblastic leukemia (ALL) receiving dapsone for pneumocystis carinii pneumonia (PCP) prophylaxis: a correlation with cytochrome b5 reductase (Cb5R) enzyme levels. Pediatr Blood Cancer. 2005;44(1):55–62.
Li S, et al. The transcriptional landscape of dorsal root ganglia after sciatic nerve transection. Sci Rep. 2015;5(1):1–13.
Starobova H, et al. Minocycline prevents the development of mechanical allodynia in mouse models of vincristine induced peripheral neuropathy. Front Neurosci. 2019;13:653.
Authier N, et al. Pain related behaviour during vincristine-induced neuropathy in rats. NeuroReport. 1999;10(5):965–8.
Boehmerle W, et al. Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice. Sci Rep. 2014;4:6370.
Fukuda Y, Li Y, Segal RA. A mechanistic understanding of axon degeneration in chemotherapy-induced peripheral neuropathy. Front Neurosci. 2017;11:481.
Soliman A, et al. Study of the possible synergistic protective effects of Melatonin and Pregabalin in Vincristine induced peripheral neuropathy Wistar Albino rats. Life Sci. 2020;244:117095.
Khalilzadeh M, et al. The protective effects of sumatriptan on vincristine-induced peripheral neuropathy in a rat model. Neurotoxicology. 2018;67:279–86.
Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci. 2017;10:174.
Yang N, et al. Protective effect of dapsone on cognitive impairment induced by propofol involves hippocampal autophagy. Neurosci Lett. 2017;649:85–92.
Ríos C, et al. Anti-apoptotic effects of dapsone after spinal cord injury in rats. Neurochem Res. 2015;40(6):1243–51.
Diaz-Ruiz A, et al. Antioxidant, anticonvulsive and neuroprotective effects of dapsone and phenobarbital against kainic acid-induced damage in rats. Neurochem Res. 2013;38(9):1819–27.
Dejban P, et al. Beneficial effects of dapsone on ischemia/reperfusion injury following torsion/detorsion in ipsilateral and contralateral testes in rat. Theriogenology. 2019;140:136–42.
Rashidian A, et al. Dapsone reduced acetic acid-induced inflammatory response in rat colon tissue through inhibition of NF-kB signaling pathway. Immunopharmacol Immunotoxicol. 2019;41(6):607–13.
Barzegar-Fallah A, et al. The neuroprotective effect of tropisetron on vincristine-induced neurotoxicity. Neurotoxicology. 2014;41:1–8.
Vera G, et al. Involvement of cannabinoid signaling in vincristine-induced gastrointestinal dysmotility in the rat. Front Pharmacol. 2017;8:37.
Ríos C, et al. Efficacy of dapsone administered alone or in combination with diazepam to inhibit status epilepticus in rats. Brain Res. 2019;1708:181–7.
Helton DR, et al. Pharmacokinetic profiles in rats after intravenous, oral, or dermal administration of dapsone. Drug Metab Dispos. 2000;28(8):925–9.
Zuidema J, Hilbers-Modderman E, Merkus F. Clinical pharmacokinetics of dapsone. Clin Pharmacokinet. 1986;11(4):299–315.
Nelson RL. The comparative clinical pharmacology and pharmacokinetics of vindesine, vincristine, and vinblastine in human patients with cancer. Med Pediatr Oncol. 1982;10(2):115–27.
Khan J, et al. Attenuation of vincristine-induced neuropathy by synthetic cyclohexenone-functionalized derivative in mice model. Neurol Sci. 2019;40:1799–811.
Farsi L, Keshavarz M, Afshari K, Javidan AN. Intravenous granulocyte colony-stimulating factor administration can attenuate neuropathic pain following spinal cord injury in male rats. Acta Med Iran. 2018;56(4):226–33.
Ja’afer FM, Hamdan FB, Mohammed FH. Vincristine-induced neuropathy in rat: electrophysiological and histological study. Exp Brain Res. 2006;173(2):334–45.
Shen H, et al. An integrated cell isolation and purification method for rat dorsal root ganglion neurons. J Int Med Res. 2019;47(7):3253–60.
Buege JA, Aust SD. [30] Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–7.
Jain P, et al. Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: prevalence and electrophysiological characteristics. J Child Neurol. 2014;29(7):932–7.
Upmanyu R, Dvivedi J, Saxena Y. Hepatotoxic effects of vincristine: an experimental study on albino rats. Indian J Physiol Pharmacol. 2009;53(3):265–70.
Geisler S, et al. Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice. Brain. 2016;139(12):3092–108.
Brigo F, et al. Vincristine-related neuropathy versus acute inflammatory demyelinating polyradiculoneuropathy in children with acute lymphoblastic leukemia. J Child Neurol. 2012;27(7):867–74.
Boyle FM, Wheeler HR, Shenfield GM. Glutamate ameliorates experimental vincristine neuropathy. J Pharmacol Exp Ther. 1996;279(1):410–5.
Cavaletti G, et al. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst. 2007;12(3):210–5.
Wang Y, et al. Sensitization of TRPV1 receptors by TNF-α orchestrates the development of vincristine-induced pain. Oncol Lett. 2018;15(4):5013–9.
Liang H, et al. Effect of NF-kB signaling pathway on the expression of MIF, TNF-α, IL-6 in the regulation of intervertebral disc degeneration. J Musculoskelet Neuronal Interact. 2018;18(4):551.
Adjuto-Saccone M, et al. TNF-α induces endothelial–mesenchymal transition promoting stromal development of pancreatic adenocarcinoma. Cell Death Dis. 2021;12(7):1–15.
Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J. 2010;277(1):2–21.
Malleo G, et al. TNF-alpha as a therapeutic target in acute pancreatitis–lessons from experimental models. Sci World J. 2007;7:431–48.
Topp KS, Tanner KD, Levine JD. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J Comp Neurol. 2000;424(4):563–76.
Kiguchi N, et al. The critical role of invading peripheral macrophage-derived interleukin-6 in vincristine-induced mechanical allodynia in mice. Eur J Pharmacol. 2008;592(1–3):87–92.
Diaz-Ruiz A, et al. Delayed administration of dapsone protects from tissue damage and improves recovery after spinal cord injury. J Neurosci Res. 2011;89(3):373–80.
Afshari K, et al. Antibiotics with therapeutic effects on spinal cord injury: a review. Fundam Clin Pharmacol. 2021;35(2):277–304.
Muthuraman A, et al. Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. Eur J Pharmacol. 2008;587(1–3):104–11.
Shati AA. Sub-chronic administration of vincristine sulfate induces renal damage and apoptosis in rats via induction of oxidative stress and activation of Raf1-MEK1/2-Erk1/2 signal transduction. Int J Morphol. 2019;37(1):273–83.
Groninger E, et al. Vincristine induced apoptosis in acute lymphoblastic leukaemia cells: a mitochondrial controlled pathway regulated by reactive oxygen species? Int J Oncol. 2002;21(6):1339–45.
Diaz-Ruiz A, Nader-Kawachi J, Calderón-Estrella F, Mata-Bermudez A, Alvarez-Mejia L, Ríos C. Dapsone, an effective neuro, and cytoprotective drug and more. Curr Neuropharmacol. 2022;20(1):194–210.
Mahale A, et al. Dapsone prolong delayed excitotoxic neuronal cell death by interacting with proapoptotic/survival signaling proteins. J Stroke Cerebrovasc Dis. 2020;29(8):104848.
Acknowledgements
We appreciate Gilaranco Co. for providing dapsone powder for this research. Special thanks go to the Mehr Laboratory for their help.
Funding
This study was supported by the National Institute for Medical Research Development [Grant No 971024] and Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shayesteh, S., Khalilzadeh, M., Takzaree, N. et al. Dapsone improves the vincristine-induced neuropathic nociception by modulating neuroinflammation and oxidative stress. DARU J Pharm Sci 30, 303–310 (2022). https://doi.org/10.1007/s40199-022-00448-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-022-00448-6