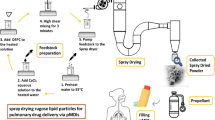
Abstract
Purpose
Spray-freeze drying (SFD) incorporating diverse carbohydrates and leucine was employed to obtain dried nanosuspension of cefixime with improved dissolution profile, good dispersibility, and excellent inhalation performance.
Methods
Nanoprecipitation was utilized to prepare nanoparticles (NPs). Nanosuspensions of cefixime were solidified via SFD to access inhalable microparticles. The aerosolization efficiencies were evaluated through twin stage impinger (TSI). Laser light scattering and scanning electron microscopy (SEM) provided assistance to determine the particle size/size distribution and morphology, respectively. Amorphous/ crystalline states of materials were examined via differential scanning calorimetry (DSC) and X-ray diffraction (XRD). Release profiles of candidate preparations were evaluated.
Results
The fine particle fraction (FPF) ranged from 18.96 ± 0.76 to 79.28 ± 0.45%. The highest value resulted from trehalose with NP/carrier ratio of 1:1 and leucine 20%. The particle size varied from 5.24 ± 0.97 to 10.17 ± 1.01 μm. The most and the least size distribution were achieved in mannitol and trehalose containing formulations, respectively. The majority of samples demonstrated ideally spherical morphology with diverse degrees of porosity and without needle-shaped structure. Percentages of release in F7 and F8 were 89.33 ± 0.88% and 93.54 ± 1.02%, respectively, via first 10 min.
Conclusion
SFD of nanosuspensions can be established as a platform for the pulmonary delivery of poorly water-soluble molecules of cefixime. Trehalose and raffinose with a lower ratio of NP to the carrier and higher level of leucine could be introduced as favorable formulations for further respiratory delivery of cefixime.
Graphical abstract





Similar content being viewed by others
References
Brogden RN, Campoli-Richards DM. Cefixime. A review of its antibacterial activity. Pharmacokinetic properties and therapeutic potential. Drugs. 1989;38(4):524–50.
Mamzoridi K, et al. Pharmacokinetics of cefixime in children with urinary tract infections after a single oral dose. Pharmacol Toxicol. 1996;78(6):417–20.
Hamilton-Miller JM. Overview of cefixime use in community-acquired infections. Clin Microbiol Infect : Off Publ Eur Soc Clin Microbiol Infect Dis. 2000;6(Suppl 3):79–81.
Genvresse I, Carbon C. Cefixime. Int J Antimicrob Agents. 1993;3(1):1–16.
Ramirez E, et al. Acceptability and characteristics of 124 human bioequivalence studies with active substances classified according to the biopharmaceutic classification system. Br J Clin Pharmacol. 2010;70(5):694–702.
Kneer J. Pharmacokinetic properties of new oral cephalosporins. Med Mal Infect. 1992;22:556–64.
Hecq J, et al. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int J Pharm. 2005;299(1–2):167–77.
Ali HS, York P, Blagden N. Preparation of hydrocortisone nanosuspension through a bottom-up nanoprecipitation technique using microfluidic reactors. Int J Pharm. 2009;375(1–2):107–13.
Niwa T, Danjo K. Design of self-dispersible dry nanosuspension through wet milling and spray freeze-drying for poorly water-soluble drugs. Eur J Pharm Sci : Off J Eur Fed Pharm Sci. 2013;50(3–4):272–81.
Wang Y, et al. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172(3):1126–41.
Wanning S, Süverkrüp R, Lamprecht A. Pharmaceutical spray freeze drying. Int J Pharm. 2015;488(1):136–53.
Bi R, et al. Spray-freeze-dried dry powder inhalation of insulin-loaded liposomes for enhanced pulmonary delivery. J Drug Target. 2008;16(9):639–48.
Mohri K, et al. Optimized pulmonary gene transfection in mice by spray–freeze dried powder inhalation. J Control Release. 2010;144(2):221–6.
Roa WH, et al. Inhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse model. J Control Release. 2011;150(1):49–55.
Rau JL. The inhalation of drugs: advantages and problems. Respir Care. 2005;50(3):367–82.
Park C-W, et al. Advanced spray-dried design, physicochemical characterization, and aerosol dispersion performance of vancomycin and clarithromycin multifunctional controlled release particles for targeted respiratory delivery as dry powder inhalation aerosols. Int J Pharm. 2013;455(1):374–92.
Chan JGY, et al. A novel dry powder inhalable formulation incorporating three first-line anti-tubercular antibiotics. Eur J Pharm Biopharm. 2013;83(2):285–92.
D’Addio SM, et al. Aerosol delivery of nanoparticles in uniform mannitol carriers formulated by ultrasonic spray freeze drying. Pharm Res. 2013;30(11):2891–901.
Rahimpour Y, Kouhsoltani M, Hamishehkar H. Alternative carriers in dry powder inhaler formulations. Drug Discov Today. 2014;19(5):618–26.
Ógáin ON, et al. Particle engineering of materials for oral inhalation by dry powder inhalers. I—particles of sugar excipients (trehalose and raffinose) for protein delivery. Int J Pharm. 2011;405(1–2):23–35.
Raula J, et al. Investigations on particle surface characteristics vs. dispersion behaviour of l-leucine coated carrier-free inhalable powders. Int J Pharm. 2010;385(1):79–85.
Cheow WS, et al. Spray-freeze-drying production of thermally sensitive polymeric nanoparticle aggregates for inhaled drug delivery: effect of freeze-drying adjuvants. Int J Pharm. 2011;404(1–2):289–300.
Molina C, Kaialy W, Nokhodchi A. The crucial role of leucine concentration on spray dried mannitol-leucine as a single carrier to enhance the aerosolization performance of albuterol sulfate. J Drug Deliv Sci Technol. 2019;49:97–106.
Leng D, et al. Formulating inhalable dry powders using two-fluid and three-fluid nozzle spray drying. Pharm Res. 2018;35(12):247.
Niwa T, Mizutani D, Danjo K. Spray freeze-dried porous microparticles of a poorly water-soluble drug for respiratory delivery. Chem Pharm Bull. 2012;60(7):870–6.
Chiang P-C, et al. Evaluation of aerosol delivery of Nanosuspension for pre-clinical pulmonary drug delivery. Nanoscale Res Lett. 2009;4(3):254–61.
Ghasemian E, et al. Optimization of cefixime nanosuspension to improve drug dissolution. Pharm Sci. 2015;21(3):136.
Alaei S, Ghasemian E, Vatanara A. Spray drying of cefixime nanosuspension to form stabilized and fast dissolving powder. Powder Technol. 2016;288:241–8.
Ogáin ON, et al. Particle engineering of materials for oral inhalation by dry powder inhalers. I-particles of sugar excipients (trehalose and raffinose) for protein delivery. Int J Pharm. 2011;405(1–2):23–35.
Parsian AR, et al. Inhalable budesonide porous microparticles tailored by spray freeze drying technique. Powder Technol. 2014;260:36–41.
Lechuga-Ballesteros D, et al. Trileucine improves aerosol performance and stability of spray-dried powders for inhalation. J Pharm Sci. 2008;97(1):287–302.
Bozdag S, et al. The effect of freeze-drying with different cryoprotectants and gamma-irradiation sterilization on the characteristics of ciprofloxacin HCl-loaded poly(D,L-lactide-glycolide) nanoparticles. J Pharm Pharmacol. 2005;57(6):699–707.
Hulse WL, et al. Influence of protein on mannitol polymorphic form produced during co-spray drying. Int J Pharm. 2009;382(1–2):67–72.
Abdelwahed W, Degobert G, Fessi H. Investigation of nanocapsules stabilization by amorphous excipients during freeze-drying and storage. Eur J Pharm Biopharm. 2006;63(2):87–94.
Pikal MJ, et al. The effects of formulation variables on the stability of freeze-dried human growth hormone. Pharm Res. 1991;8(4):427–36.
Torrado S, Torrado S. Characterization of physical state of mannitol after freeze-drying: effect of acetylsalicylic acid as a second crystalline cosolute. Chem Pharm Bull (Tokyo). 2002;50(5):567–70.
Rahmati MR, et al. Effect of formulation ingredients on the physical characteristics of salmeterol xinafoate microparticles tailored by spray freeze drying. Adv Powder Technol. 2013;24(1):36–42.
Singh BK, et al. Study on gamma and electron beam sterilization of third generation cephalosporins cefdinir and cefixime in solid state. Radiat Phys Chem. 2010;79(10):1079–87.
Bhaskar R, Patil P. Nanocrystal suspension of cefixime trihydrate preparation by high-pressure homogenization formulation design using 23 factorial design. Int J Pharm Pharm Sci. 2017;9:64.
Hooton JC, Jones MD, Price R. Predicting the behavior of novel sugar carriers for dry powder inhaler formulations via the use of a cohesive-adhesive force balance approach. J Pharm Sci. 2006;95(6):1288–97.
Chen L, et al. Amorphous powders for inhalation drug delivery. Adv Drug Deliv Rev. 2016;100:102–15.
Schoubben A, et al. Capreomycin inhalable powders prepared with an innovative spray-drying technique. Int J Pharm. 2014;469(1):132–9.
Prime D, et al. Review of dry powder inhalers. Adv Drug Deliv Rev. 1997;26(1):51–8.
Maa Y-F, et al. The effect of operating and formulation variables on the morphology of spray-dried protein particles. Pharm Dev Technol. 1997;2(3):213–23.
Bosquillon C, et al. Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. J Control Release. 2001;70(3):329–39.
Yu H, et al. Dry powder inhaler formulation of high-payload antibiotic nanoparticle complex intended for bronchiectasis therapy: spray drying versus spray freeze drying preparation. Int J Pharm. 2016;499(1–2):38–46.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haghighi, D.M., Faghihi, H., Darabi, M. et al. Spray freeze drying to solidify Nanosuspension of Cefixime into inhalable microparticles. DARU J Pharm Sci 30, 17–27 (2022). https://doi.org/10.1007/s40199-021-00426-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-021-00426-4