Abstract
Background
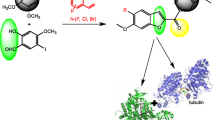
During recent years, a number of anti-tubulin agents were introduced for treatment of diverse types of cancer. Despite their potential in the treatment of cancer, drug resistance and adverse toxicity, such as peripheral neuropathy, are some of the negative effects of anti-tubulin agents. Among anti-tubulin agents, indibulin was found to cause minimal peripheral neuropathy. Thus far, however, indibulin has not entered clinical usage, caused in part by its poor aqueous solubility and other developmental problems in preclinical evaluation.
Objectives
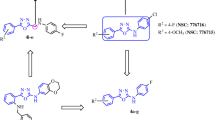
With respect to need for finding potent and safe anticancer agents, in our current research work, we synthesized several indibulin-related diarylpyrrole derivatives and investigated their anti-cancer activity.
Methods
Cell cultur studies were perfomred using the MTT cell viability assay on the breast cancer cell lines MCF-7, T47-D, and MDA-MB231 and also NIH-3 T3 cells as representative of a normal cell line. The activity of some of the synthesized compounds for tubulin interaction was studied using colchicine binding and tubulin polymerization assays. The annexin V-FITC/PI method and flow cytometric analysis were used for studying apoptosis induction and cell cycle distribution.
Results and conclusion
Two of the synthesized compounds, 4f and 4 g, showed high activity on the MDA-MB231 cell line (IC50 = 11.82 and 13.33 μM, (respectively) and low toxicity on the normal fibroblast cells (IC50 > 100 μM). All of the tested compounds were more potent on T47-D cancer cells and less toxic on NIH-3 T3 normal cells in comparison to reference compound, indibulin. The tubulin polymerization inhibition assay and [3H]colchicine binding assay showed that the main mechanism of cell death by the potent synthesized compounds was not related to an interaction with tubulin. In the annexin V/PI staining assay, the induction of apoptosis in the MCF-7 and MDA-MB231 cell lines was observed. Cell cycle analysis illustrated an increased percentage of sub-G-1 cells in the MDA-MB231 cell line as a further indication of cell death through induction of apoptosis.

Novel Indibulin analogous as anti-breast cancer agents







Similar content being viewed by others
References
Santos CCF, Paradela LS, Novaes LFT, Dias SMG, Pastre JC. Design and synthesis of cenocladamide analogues and their evaluation against breast cancer cell lines. Med Chem Commun. 2017;8:755–66.
Yao N, Chen CY, Wu CY, Motonishi K, Kung HJ, Lam KS. Novel flavonoids with antiproliferative activities against breast cancer cells. J Med Chem. 2011;54:4339–49.
Ananda Kumar CS, Nanjunda Swamy S, Thimmegowda NR, Benaka Prasad SB, Yip GW, Rangappa KS. Synthesis and evaluation of 1-benzhydryl-sulfonylpiperazine derivatives as inhibitors of MDA-MB-231 human breast cancer cell proliferation. Med Chem Res. 2007;16:179–87.
Gautam Y, Dwivedi S, Srivastava A, Hamidullah H, Singh A, Chanda D, et al. 2-(3′, 4′-dimethoxybenzylidene) tetralone induces anti-breast cancer activity through microtubule stabilization and activation of reactive oxygen species. RSC Adv. 2016;6:33369–79.
Bass R, Jenkinson S, Wright J, Srinivasan TS, Marshall JC, Castagnolo D. Synthesis and biological evaluation of novel amidinourea and triazine congeners as inhibitors of MDA-MB-231 human breast cancer cell proliferation. Chem Med Chem. 2017;12:288–91.
Romagnoli R, Baraldi PG, Salvador MK, Preti D, Tabrizi MA, Brancale A, et al. Synthesis and evaluation of 1,5-disubstituted tetrazoles as rigid analogues of combretastatin A-4 with potent antiproliferative and antitumor activity. J Med Chem. 2012;55:475–88.
Lu Y, Chen J, Xiao M, Li W, Miller DD. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res. 2012;29:2943–71.
Wienecke A, Bacher G. Indibulin, a novel microtubule inhibitor, discriminates between mature neuronal and nonneuronal tubulin. Cancer Res. 2009;69:171–7.
Colley HE, Muthana M, Danson SJ, Jackson LV, Brett ML, Harrison J, et al. An orally bioavailable, indole-3-glyoxylamide based series of tubulin polymerization inhibitors showing tumor gGrowth inhibition in a mouse xenograft model of head and neck cancer. J Med Chem. 2015;58:9309–33.
Marchand P, Antoine M, Le Baut G, Czech M, Baasner S, Günther E. Synthesis and structure–activity relationships of N-aryl(indol-3-yl)glyoxamides as antitumor agents. Bioorg Med Chem. 2009;17:6715–27.
Li WT, Hwang DR, Chen CP, Shen CW, Huang CL, Chen TW, et al. Synthesis and biological evaluation of N-heterocyclic indolyl glyoxylamides as orally active anticancer agents. J Med Chem. 2003;46:1706–15.
Fang Z, Liao PC, Yang YL, Yang FL, Chen YL, Lam Y, et al. Synthesis and biological evaluation of polyenylpyrrole derivatives as anticancer agents acting through caspases-dependent apoptosis. J Med Chem. 2010;53:7967–78.
Carbone A, Pennati M, Parrino B, Lopergolo A, Barraja P, Montalbano A, et al. Novel 1h-pyrrolo[2,3-b]pyridine derivative nortopsentin analogues: synthesis and antitumor activity in peritoneal mesothelioma experimental models. J Med Chem. 2013;56:7060–72.
Wang S, Wood G, Meades C, Griffiths G, Midgley C, McNae I, et al. Synthesis and biological activity of 2-anilino-4-(1H-pyrrol-3-yl) pyrimidine CDK inhibitors. Bioorg Med Chem Lett. 2004;14:4237–40.
Ghasemi M, Ghadbeighi S, Amirhamzeh A, Tabatabai SA, Ostad SN, Shafiee A, et al. Synthesis, molecular docking study, and cytotoxic activity of 1,3,5-triaryl pyrazole derivatives. Lett Drug Des Discov. 2016;13:121–8.
Ghadbegi S, Ostad SN, Shafiee A, Amini M. Synthesis and anticancer activity of 1,3,5-triaryl-1H-pyrazole. Lett Drug Des Discov. 2015;12:754–9.
Miralinaghi P, Salimi M, Amirhamzeh A, Norouzi M, Kandelousi HM, Shafiee A, et al. Synthesis, molecular docking study, and anticancer activity of triaryl-1,2,4-oxadiazole. Med Chem Res. 2013;22:4253–62.
Salehi M, Ostad SN, Riazi GH, Assadieskandar A, Shavi TC, Shafiee A, et al. Synthesis, cytotoxic evaluation, and molecular docking study of 4,5-diaryl-thiazole-2-thione analogs of combretastatin A-4 as microtubule-binding agents. Med Chem Res. 2014;23:1465–73.
Zareian B, Ghadbeighi S, Amirhamzeh A, Ostad SN, Shafiee A, Amini M. Synthesis, molecular docking study, and cytotoxic activity of 3,4-diaryl-5-(4-pyridinyl)-1,2,4-oxadiazole. Med Chem. 2016;12:394–401.
Saravani F, Saeedian Moghadam E, Salehabadi H, Ostad SN, Pirali Hamedani M, Amanlou M, et al. Synthesis, anti-proliferative evaluation, and molecular docking studies of 3-(alkylthio)-5,6-diaryl-1,2,4-triazines as tubulin polymerization inhibitors. Lett Drug Des Discov. 2018;15. https://doi.org/10.2174/1570180815666180727114216.
Saeedian Moghadam E, Saravani F, Ostad SN, Tavajohi S, Pirali Hamedani M, Amini M. Design, synthesis and cytotoxicity evaluation of indibulin analogs. Heterocycl Commun. 2018;24:211–8.
Peloquin AJ, Stone RL, Avila SE, Rudico ER, Horn CB, Gardner KA, et al. Synthesis of 1,3-diphenyl-6-alkyl/aryl-substituted fulvene chromophores: observation of π–π interactions in a 6-pyrene-substituted 1,3-diphenylfulvene. J Org Chem. 2012;77:6371–6.
Stetter H. Addition von aromatischen und heterocyclischen Aldehyden an α, â unsaturated ketone. Chem Ber. 1974;107:2453–8.
Saeedian Moghadam E, Amini M. 2-[2-Methyl-5-phenyl-1-(3,4,5 trimethoxyphenyl) 1Hpyrrol-3-yl]-2-oxo-N-(pyridin-4-yl) acetamide. Molbank. 2018;2018. https://doi.org/10.3390/M1002.
Hamel E, Lin CM. Separation of active tubulin and microtubule-associated proteins by ultracentrifugation and isolation of a component causing the formation of microtubule bundles. Biochem. 1981;23:4173–84.
Hamel E, Lin CM. Stabilization of the colchicine-binding activity of tubulin by organic acids. Biochim Biophys Acta. 1984;675:226–31.
Verdier-Pinard P, Lai JY, Yoo HD, Yu J, Marquez B, Nagle DG, et al. Structure-activity analysis of the interaction of Curacin a, the potent colchicine site antimitotic agent, with tubulin and effects of analogs on the growth of MCF-7 breast cancer cells. Mol Pharmacol. 1998;53:62–76.
Hamel E. Evaluation of antimitotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem Biophys. 2003;38:1–22.
Assadieskandar A, Amini M, Ostad SN, Riazi GH, Shavi TC, Shafiei B, et al. Design, synthesis, cytotoxic evaluation and tubulin inhibitory activity of 4-aryl-5-(3,4,5-trimethoxyphenyl)-2-alkylthio-1H-imidazole derivatives. Bioorg Med Chem. 2013;21:2703–9.
Bacher G, Nickel B, Emig P, Vanhoefer U, Seeber S, Shandra A, et al. D-24851, a novel synthetic microtubule inhibitor, exerts curative antitumoral activity in vivo, shows efficacy toward multidrug-resistant tumor cells, and lacks neurotoxicity. Cancer Res. 2001;61:392–9.
Acknowledgements
This work was financially supported by Research Council of Tehran University of Medical Sciences.
The content of this paper is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saeedian Moghadam, E., Hamel, E., Shahsavari, Z. et al. Synthesis and anti-breast cancer activity of novel indibulin related diarylpyrrole derivatives. DARU J Pharm Sci 27, 179–189 (2019). https://doi.org/10.1007/s40199-019-00260-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-019-00260-9