Abstract
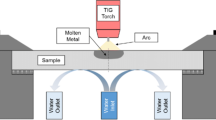
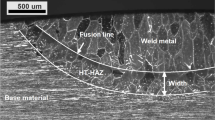
This study deals with the selection of appropriate welding parameters during autogenous arc welding of duplex stainless steels in order to achieve an optimum phase balance of austenite and ferrite in the as-welded microstructure. Specimens of duplex stainless steel 2205 with dimensions (40 × 40 × 10) mm3 were welded using autogenous arc welding under 95% Ar + 5% vol. N2 atmosphere. The weld pool temperature was measured by non-contact infrared temperature measurement, the weld bead dimensions were determined using scanning electron micrographs, and the final nitrogen concentration was evaluated by optical emission spectroscopy. The kinetics of nitrogen absorption and desorption in molten duplex stainless steel was discussed and all the relevant variables were presented. The effect of welding current and speed on the final nitrogen concentration was also discussed. Finally, based on this analysis, a method was set up which can be used to optimize the phase balance by using predictive methods of the Ferrite Number, such as the Welding Research Council (WRC)-92 diagram.



Similar content being viewed by others
Abbreviations
- \( \frac{d{m}_N}{dt} \) :
-
rate of mass transfer of nitrogen (kg s−1)
- Κa :
-
rate constant for the reaction of absorption of monatomic nitrogen in a stainless steel melt (kg m−2 s−1 atm−1)
- A:
-
weld pool surface area (m2)
- N(g) :
-
the monatomic nitrogen content of the arc (atm)
- Neq(g) :
-
the monatomic nitrogen content of the arc plasma required for equilibrium with the nitrogen in the steel (atm)
- ρ :
-
the density of the molten weld metal (kg m−3)
- V :
-
the weld pool volume (m3)
- α :
-
weld pool radius (m)
- h :
-
weld pool depth (m)
- f N, T :
-
the activity coefficient of nitrogen in the stainless steel melt at temperature T
- e N X :
-
first-order interaction parameter of nitrogen
- r N X :
-
second-order interaction parameter of nitrogen
- Ni :
-
the initial nitrogen concentration of the stainless steel
- X ref eq :
-
wt% equivalent concentration of element i (the content of the reference element which provides the same nitrogen activity as the content of element i)
- c ref i :
-
equivalent factor
- X i :
-
wt% concentration of alloying element i in stainless steel
- ΔΤ :
-
difference between the weld pool temperature and the melting point of the stainless steel
- v :
-
welding speed (m/s)
- L :
-
weld pool diameter (m)
- Κ d :
-
rate constant for the reaction of nitrogen desorption from the weld pool to the arc atmosphere (kg m−2 s−1 (wt%)−2)
- Nsteel :
-
the final nitrogen concentration in the solidified steel (wt%)
- Neq :
-
nitrogen concentration in the weld pool for equilibrium with the nitrogen in the gas (wt%)
- f o :
-
the activity coefficient of oxygen in the stainless steel melt
- f s :
-
the activity coefficient of sulfur in the stainless steel melt
- [%Ο]:
-
wt% concentration of oxygen in the weld pool
- [%S]:
-
wt% concentration of sulfur in the weld pool
- T d :
-
dissociation temperature of diatomic to monatomic nitrogen
- T :
-
weld pool temperature
References
Gunn R (1997) Duplex stainless steels: microstructure, properties and applications. 1st ed. Cambridge, Woodhead Publishing Ltd
Liljas M (1994) The welding metallurgy of duplex stainless steels. In: Gooch TG, editor. Proceedings of the Fourth International Conference on Duplex Stainless Steels, Glasgow, Scotland, Keynote Paper V, vol. 2, 13–16
Terasaki T, Gooch TG (1995) Prediction of cooling time for ferrite-austenite transformation in duplex stainless steel. ISIJ Int 35(10):1272–1276
Nowacki J, Łukojc A (2006) Microstructural transformations of heat affected zones in duplex steel welded joints. Mater Charact 56(4–5):436–441
Yang Y, Yan B, Li J, Wang J (2011) The effect of large heat input on the microstructure and corrosion behaviour of simulated heat affected zone in 2205 duplex stainless steel. Corros Sci 53(11):3756–3763
Xavier CR, Campos MF, Castro JA (2013) Numerical method applied to duplex stainless steel welding. Ironmak Steelmak 40(6):420–429
Blom KJ. Improving properties of weld joints in duplex stainless steel by welding with shielding gas containing nitrogen. In: Stainless Steels’87, York, UK, Institute of Metals, 123–5
Muthupandi V, Srinivasan PB, Seshadri SK, Sundaresan S (2003) Corrosion behaviour of duplex stainless steel weld metals with nitrogen additions. Corros Eng Sci Technol 38(4):303–308
Muñoz AI, Antón JG, Guiñón JL, Herranz VP (2005) Effect of nitrogen in argon as a shielding gas on tungsten inert gas welds of duplex stainless steels. Corros 61(7):693–705
Bhatt RB, Kamat HS, Ghosal SK, De PK (1999) Influence of nitrogen in the shielding gas on corrosion resistance of duplex stainless steel welds. J Mater Eng Perform 8(5):591–597
Wiktorowicz R, Crouch J (1996) Shielding gas developments for TIG welding of duplex and super duplex stainless steels. Weld Res Abroad 42(4):33–34
Hertzman S, Charles J (2011) On the effect of nitrogen on duplex stainless steels. Rev Métall 108(7–8):413–425
Karlsson L (2012) Welding duplex stainless steels - a review of current recommendations. Weld World 56(5):65–76
Messer B, Oprea V, Wright A (2007) Duplex stainless steel welding: best practices. Stainl Steel World:53–63
Kannan T, Murugan N (2006) Prediction of ferrite number of duplex stainless steel clad metals using RSM. Weld J 85(5):91–100
Magnabosco R, dos Santos DC (2012) Intermetallic phases formation during short aging between 850 o C and 950 o C of a superduplex stainless steel. J Mater Res Technol 1(2):71–74
Rokanopoulou A, Papadimitriou GD (2011) Titanium carbide/duplex stainless steel (DSS) metal matrix composite coatings prepared by the plasma transferred arc (PTA) technique: microstructure and wear properties. J Coat Technol Res 8(3):427–437
Du Toit M, Pistorius PC (2003) Nitrogen control during the autogenous arc welding of stainless steel. Part 2: a kinetic model for nitrogen absorption and desorption. Weld J 82(9):231–237
DebRoy T, David SA (1995) Physical processes in fusion welding. Rev Mod Phys 67(1):85–112
Gedeon SA, Eagar TW (1990) Thermochemical analysis of hydrogen absorption in welding. Weld J 69(7):264–271
Kou S (2003) Welding metallurgy. 1st ed. Wiley-Interscience, Hoboken, pp 68–71
Kubaschewski O, Alcock C, Spencer P (1993) Materials thermochemistry. 1st ed. Pergamon, Oxford
Palmer TA, DebRoy T (2000) Numerical modeling of enhanced nitrogen dissolution during gas tungsten arc welding. Metall Mater Trans B 31(6):1371–1385
Wagner C (1952) Thermodynamics of alloys. 1st ed. Reading, Ma Addison-Wesley Publishing Co., Reading, Massachusetts
Forch K, Stein G, Menzel J (1990) Technologies of newly developed high-nitrogen steels, In: “Proceedings of the Third International Conference on High-Nitrogen Steels (Aachen, Germany, October 1990), G. Stein, H. Witulski (eds.), Stahleisen, p. 258–267
Elliott J, Gleiser M, Ramakrishna V (1963) Thermochemistry for steelmaking. 1st ed. Addison-Wesley, Reading, p 75
Wada H, Pehlke RD (1977) Solubility of nitrogen in Fe-Cr-Ni alloys containing manganese and molybdenum. Metall Trans B 8(4):675–682
Mizukami H, Shirai Y, Yamanaka A, Watanabe T (2000) Prediction of density of stainless steel. ISIJ Int 40(10):987–994
Sandvik Materials Technology (2008) Datasheet S -1874 – Eng
Bermejo MAV (2012) Predictive and measurement methods for delta ferrite determination in stainless steels. Weld J 91(4):113–121
Lundin CD, Zhou G, Ruprecht W (1999) Ferrite measurement in austenitic and duplex stainless steel castings - final report (No. DOE/ID/13734-1). USDOE Idaho Operations Office, Idaho Falls (ID); The University of Tennessee, Knoxville (TN)
Kotecki DJ, Siewert TA (1992) WRC-1992 constitution diagram for stainless steel weld metals: a modification of the WRC-1988 diagram. Weld J 71(5):171–178
Acknowledgements
The State Scholarships Foundation of Hellas (IKY) is gratefully acknowledged for the financial support to A. Rokanopoulou (Grant No. 4327) and P. Skarvelis (Grant No. 4246).
Author information
Authors and Affiliations
Corresponding author
Additional information
Recommended for publication by Commission XV - Design, Analysis, and Fabrication of Welded Structures
Rights and permissions
About this article
Cite this article
Rokanopoulou, A., Skarvelis, P. & Papadimitriou, G.D. Welding design methodology for optimization of phase balance in duplex stainless steels during autogenous arc welding under Ar–N2 atmosphere. Weld World 63, 3–10 (2019). https://doi.org/10.1007/s40194-018-0660-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-018-0660-0