Abstract
Purpose of Review
Present the state-of-the-art overview of laryngeal pacing for treatment of bilateral vocal fold paralysis. A minimally invasive unilateral pacing system and a fully implantable bilateral pacing system are currently in clinical trials. The relative advantages and disadvantages of each are discussed.
Recent Findings
Research in functional electrical stimulation for the reanimation of the posterior cricoarytenoid muscle has successfully translated from animal models to human clinical trials for unilateral pacing and bilateral pacing. Current findings suggest unilateral pacing in humans significantly improves ventilation but only marginally better than cordotomy. Bilateral pacing in canines increases glottal opening greater than 2-fold over unilateral pacing and restores exercise tolerance to normal.
Summary
Unilateral pacing can be considered a breathing assist device and may not be appropriate for active individuals. Bilateral pacing may be preferable for patients who wish to engage in strenuous exercise. Minimally invasive systems may be ideal for patients who prefer less invasive implantation and are not concerned with cosmesis. Fully implantable pacing systems offer greater electrode redundancy and stability, resulting in a system that is robust against electrode migration or damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vocal fold motion impairment (VFMI) describes the phenomenon of partial or complete disruption of vocal fold abduction and/or adduction during the respiration/phonation cycle. Importantly, VFMI is a symptom of an underlying disease process rather than a specific diagnosis. Characterization of the underlying etiology and pathophysiology is critical for clinical decision making. VFMI may result from mechanical or neurological disruption of the laryngeal mechanism. In particular, vocal fold paralysis is a form of VFMI caused by lesion(s) of the recurrent laryngeal nerve (RLN), resulting in hypomobility (paresis) or immobility (paralysis) of the vocal folds either unilaterally or bilaterally.
While both unilateral vocal fold paralysis (UVFP) and bilateral vocal fold paralysis (BVFP) are of medical concern, BVFP is a serious and often life-threatening clinical condition. Depending on the glottal configuration of the vocal folds, voicing may be functional, but airway compromise is often severe enough to warrant at least a temporary tracheostomy to relieve inspiratory stridor and dyspnea [1,2,3].
Once the airway has been stabilized, the long-term goal of intervention is to recover ventilation through enlarging the airway either statically or dynamically. Historically, procedures such as arytenoidectomy and cordotomy have been regarded as the standard of care for airway enlargement. However, since these structurally based interventions can cause irreversible damage to the voice and compromise airway protection during swallowing, they should only be considered in case of PCA denervation and irreversible muscle atrophy [4•]. Findings from basic research suggest the appropriateness of these interventions to increase the airway are more limited than previously thought. It is a common misconception that vocal fold paralysis implies complete PCA denervation; rather, denervation is just one of multiple underlying mechanisms resulting in vocal fold paralysis. Animal and human electromyography studies have found the RLN reinnervates laryngeal muscles in the large majority of subjects [1, 5,6,7,8], despite complete RLN resection [9].
Whereas these findings highlight the persistence of the peripheral nervous system to regenerate, incomplete or aberrant neural regeneration results in suboptimal clinical outcomes. Siribodhi et al. [7] was the first to describe aberrant reinnervation of muscle fibers as a potential explanation for vocal fold paralysis following RLN injury. Crumley [10] termed this phenomenon “laryngeal synkinesis”. The RLN carries motor fibers that innervate both the abductor (posterior cricoarytenoid, PCA) muscle and adductor muscles of the vocal folds. Damage to the nerve compromises both of these functions and arrests the vocal folds in a near-closed position. If the structural integrity of a nerve is damaged to the extent that the continuity of the endoneurial tube is compromised, as with neurotmesis [11], regenerating axons may exit their native endoneurial conduits, enter surrounding endoneurial tubes, and randomly reinnervate nonnative muscle fibers. The functional result is a coupling of disparate muscles to the impulses of aberrant motoneurons. Since there are more adductor muscles than abductor muscles, the nerve fibers of each motoneuron type are 4 times more likely to reinnervate adductors. When the neural impulse intended for glottal opening via PCA activation is rerouted to adductor muscles, paradoxical closure can occur. As a result of synkinetic reinnervation, the vocal folds stay partially or fully closed at rest, and during more strenuous activity, they close even further. It is estimated that at least 66–88% of BVFP patients are synkinetically reinnervated on at least one side following RLN injury [12].
Theoretical Basis for Laryngeal Pacing
Laryngeal pacing is the application of functional electrical stimulation (FES) of the PCA muscle. It is a more dynamic and physiologic treatment approach than static airway enlargement procedures. The fundamental principle is that following nerve damage, muscles can be reanimated with FES controlled by an external sensor. Ideally, stimulation would be triggered synchronously with the inspiratory cycle; however, in practice, both animal and human studies have shown that laryngeal pacing is effective without inspiratory triggering.
FES has been found to be highly effective in case of synkinetic reinnervation, where muscle activation occurs indirectly through stimulation of regenerated RLN nerve branches or terminals in the PCA muscle. Axon activation is more efficient than direct muscle fiber activation because of the lower capacitance and effective charging of an axon to firing. Further, when a single motor unit axon is stimulated, multiple muscle fibers are spatially recruited because of its terminal branching. In contrast, FES of denervated muscle is possible but inefficient. Direct muscle activation requires greater charging and necessitates activation of individual muscle fibers in the absence of spatial recruitment.
Application of FES to paralyzed laryngeal muscles was conceived and introduced into the field of otolaryngology in 1977 by Zealear and Dedo [13]. They demonstrated that a unilaterally paralyzed axial (laryngeal, facial, pharyngeal, extraocular, etc.) muscle could be reanimated by electrical stimulation, via control signals relayed from its contralateral partner. While the experimental model was the cricothyroid muscle of the canine, Zealear [13, 14] proposed that FES might also be useful in the “restoration of motion of bilaterally paralyzed vocal cords, paralyzed or uncoordinated pharynx muscles used for swallowing, as well as other paralyzed muscles of the head, neck, and thorax.”
Unilateral Laryngeal Pacing
Since its introduction, numerous studies of laryngeal reanimation have been conducted in acute animal preparations. Obert et al. [15] first demonstrated the feasibility of electrically stimulated glottal opening. They restored complete bilateral abduction in acutely paralyzed canines via paced stimulation of the PCA muscle. They also postulated that paced stimulation could be effectively synchronized with inspiration. In the 1980s, Bergmann [16, 17] first demonstrated the plausibility of synchronized laryngeal pacing, using a chest wall sensor to trigger stimulation of abductors in a canine UVFP model. Further progress toward laryngeal pacing was realized in a series of acute studies by Sanders [18], who systematically evaluated the stimulus-response characteristics of chemically denervated canine abductor muscle and obtained the dynamic range of parameters effective for PCA muscle activation.
The evolution of an implantable laryngeal stimulation device began as early as 1986 when Zrunek et al. [19] described continuous pulsed stimulation of the denervated PCA muscle in sheep using a comb-shaped array of four bipolar electrodes. Muscle contractility and evoked abduction were restored to normal values with stimulation over a 2-month period. In the 1990s, Zealear et al. [20] fabricated and employed a prototype circuit for stimulation of the chronically denervated (UVFP) canine larynx. In this study, stimuli were generated by a circuit affixed to the skull and delivered through an electrode patch array, placed on the surface of the PCA muscle. The stimulus paradigm suitable for reanimation of the chronically denervated PCA muscle was identified. Chronic stimulation was found to restore abduction, increase PCA muscle contractility over the period of intervention, and protect the muscle from atrophy and fibrosis [21].
Such animal studies of abductor pacing have provided a foundation for subsequent human trials. Bergmann reported development of an implantable laryngeal pacing system for potential use in human in the 1980s; however, human implantation was not pursued due to the identification of unspecified technical limitations of the implants [22]. Zealear et al. [23] performed the first study of human laryngeal pacing in 1995 using an external pacing circuit and percutaneous needle electrode temporarily inserted into the PCA muscle of patients with unilateral laryngeal paralysis. Stimulation was triggered by inspiratory chest wall expansion using a plethysmography transducer. This was the first study providing proof of concept of laryngeal pacing in human; however, the focus was not to treat unilateral vocal fold paralysis. Subsequently, the first clinical trial of laryngeal pacing in BVFP patients was conducted in Europe and the USA by Zealear’s team [24] using a totally implantable, commercially available stimulator (Itrel II™, Medtronic, Inc.), originally designed for pain control. Stimulation was applied to one side of the larynx, unsynchronized with respiration. During the trial, Zealear et al. [24] discovered a technical challenge repurposing this commercially available device for laryngeal pacing. Specifically, anode corrosion occurred between 6 and 30 months after device activation in 3 of 7 patients. Switching from bipolar to monopolar stimulation using the IPG case (with larger surface area) as the anode mitigated corrosion for the remainder of the study [24]. Parenthetically, the monopolar format was more efficient, conserving battery charge with less intercontact current shunting. Subsequent bench testing of the Itrel II™ revealed the electronic charge balancing system had the limitation of producing anode corrosion at the longer pulse durations required for efficient PCA stimulation. Zealear discontinued use of the Itrel II™ for future animal and human studies and used implantable systems with capacitively coupled, charge balancing circuits instead.
Despite this technical challenge, the results of this clinical trial demonstrated the clear potential merit of unilateral laryngeal pacing as a new treatment modality for chronic BVFP. Six of seven patients were implanted successfully. Five patients showed stimulated abduction on the implanted side. Peak inspiratory flow (PIF) improved in these 5 patients, with oral ventilation sufficient for decannulation. Further, despite the lack of inspiratory synchronization, there were no deleterious effects of laryngeal pacing on voice, and there was no case of aspiration in any patient over more than 12 months of study [25]. Voice and ventilation outcomes from these patients were later compared with cordotomy patients. Pacing patients had statistically significantly greater ventilation and voice quality outcomes than cordotomy, demonstrating the superiority of unilateral pacing; however, it should be noted that the improvement with unilateral pacing was only marginally better [26]. Although synchronized stimulation with inspiration would be ideal, it appeared unnecessary from this study. All but one patient had synkinetic reinnervation of the adductors sufficient to maintain a closed position of the vocal folds during swallowing and voicing, overriding any stimulated opening of the airway. The remaining patient had mild breathy voice, but swallowing was not affected [25].
In 2013, Mueller and colleagues developed a novel, minimally invasive laryngeal pacing system (referred to as the LP System) utilizing a miniaturized, bipolar, spiral tip electrode (MED-EL, Innsbruck, Austria) to deliver stimulation. The LP electrode was inserted into the PCA of normally innervated mini pigs using a custom, hollow-needle insertion tool. It was advanced percutaneously through the anterior neck, through the subglottic soft tissue space of the larynx, and into the ventral aspect of the cricoid lamina. Using small drilling movements, the tool was further advanced through the cricoid lamina into the PCA. Electrode placement was then tested and revised until a “hot spot” was identified which generated vocal fold abduction without coactivation of adductors. Then the insertion tool was removed, and the electrode was fixated to the ventral aspect of the cricoid arch [27]. In order to deliver stimulation, Förster et al. [28] then connected the electrode to an external digital interface box (MED-EL) and tested the integrity and stimulation capacity of the implant in normally innervated mini pigs.
In 2016, a second-generation version of this MED-EL LP System was tested in a clinical trial for patients with BVFP. The electrodes were inserted as previously described; however, the electrode leads were tunneled subcutaneously to a pocket located in the upper sternum region and connected to an LP implant containing an inductive receiver coil. An external LP processor containing an inductive transmission coil with pulse generator and battery was magnetically attached to the implanted receiver through the skin, and programmed stimulation was delivered transcutaneously. Implantation was successful in 8/9 patients, and repositioning of the electrodes was successful in mitigating adverse effects such as electrode dislocation or patient-reported discomfort in the neck and chest [29]. Ventilatory measures of peak expiratory flow (PEF) and absolute PIF improved significantly 6-month post-implantation [29]. Patients showed improved range in vocal intensity at 6-months post-op, while voice perturbation measures, fundamental frequency, and maximum phonation time remained unchanged. Swallowing was unaffected by stimulation [30•].
The significant advantage of the MED-EL system is its innovative electrode design. The minimally invasive insertion technique was devised to reduce the risk of further RLN damage or excessive tissue scarring associated with implantation via neck dissection. Förster et al. [27] reported negligible laryngeal swelling in mini pigs in the immediate postoperative period, attributed to the reduced tissue trauma caused by these minimally invasive surgical procedures. Conversely, the potential disadvantage of the MED-EL system is the lack of redundancy in electrode contacts. Even slight migration of the electrode could lead to loss of abductory response, necessitating reimplantation. Further, the spiral tip of the electrode serving as the lead’s anchor in the muscle may be insufficient to prevent migration. There is also concern regarding the lead’s susceptibility to damage at its site of passage through the cricoid cartilage in face of movement with development of work hardness. It is possible that although the implantation procedure is minimally invasive, more repositioning and/or reimplantation procedures may be necessary to maintain consistent therapeutic effect. Finally, the LP processor is not an implantable device and must be worn externally on the sternum. Whereas this external placement is advantageous for changing the processor battery, it may be cosmetically unappealing and susceptible to inadvertent detachment from the chest wall.
Bilateral Laryngeal Pacing
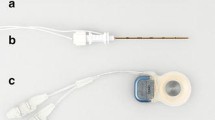
Zealear’s team continued their line of research testing a series of deep brain stimulators (DBS) implanted into the PCA muscles bilaterally in a canine BVFP model. The Genesis XP™ (St. Jude Medical-Neuro Division, Inc., Plano, TX) 4-channel, bipolar DBS electrodes were selected due to their closely spaced channel contacts. These electrodes were size-appropriate for implantation into the PCA, and the 4-channel contact configuration provided channel redundancy in case of electrode migration. Unlike the MED-EL pacing system, electrode implantation required neck dissection. The trachea was dissected free from the esophagus, and the inferior border of the cricoid cartilage was exposed. On each side, a submuscular pocket was created between the PCA muscle and the underlying cricoid cartilage using a periosteal elevator. A DBS electrode was inserted 14.5 mm into each pocket halfway between the point of RLN entry and the median raphe, with a trajectory parallel to the midline (Fig. 1a). After endoscopic confirmation that stimulation produced vocal fold abduction for each channel, the electrodes were anchored to the cricoid cartilage at the inferior border of the PCA. The channels were numbered 1 to 4 on the left side and 5 to 8 on the right side from tip to base of each electrode (Fig. 1b). The electrode leads were interfaced with an implantable pulse generator (IPG), which was positioned in a submuscular pocket beneath the trapezius muscle (Fig. 1c). After implantation, IPG stimulus parameters could be changed transcutaneously with an external programmer through a radio frequency link [31, 32].
a Posterior view of the canine larynx showing the deep brain stimulation (DBS) electrode inserted into the submuscular pocket of the right posterior cricoarytenoid (PCA) muscle. Detail of the left PCA muscle electrode can be appreciated prior to insertion. b Dimensions of deep brain stimulation electrodes. Both electrodes have four channel contacts on each side (Ch 1–4 on the left, Ch 5–8 on the right) inserted 14.5 mm into the PCA muscle. c DBS electrodes used for bilateral PCA stimulation. DBS electrodes are interfaced with an implantable pulse generator (St. Jude Medical-Neuro Division, Inc., Plano, TX)
Four animals were implanted with the Genesis XP™ system [31]. This canine study demonstrated the capacity of bilateral pacing to generate significantly greater abduction than unilateral pacing [32]. Bilaterally stimulated glottal area was found to be more than double (~ 3×) the unilaterally stimulated glottal area (unpublished observations). This can be explained by the fact that during unilateral stimulation the passive vocal fold is drawn toward the midline via linkage to the active side, partially obstructing the airway. In view of the large glottal areas achieved by bilateral stimulation, it was not unexpected to observe that exercise tolerance was restored to normal baseline levels in these animals [31]. With the stimulator off, animals could only ambulate on the treadmill without becoming dyspneic. After turning the device on for bilateral stimulation, animals were able to complete the entire treadmill test, running for 12 min at speeds increasing to 8 miles/h at 3-min intervals. If the stimulation was restricted to one side, the animals were unable to complete the full treadmill test [33]. These remarkable outcomes underscore the potential significance of bilateral laryngeal pacing for the treatment of BVFP. A long-term follow-up study demonstrated that these animals maintained their functional ventilation gains without impairing swallowing function for the entire study, up to 20 months postoperatively [34].
St. Jude has since discontinued the Genesis XP™ device; however, their next generation Infinity™ DBS 8-channel electrodes and IPG offer even greater flexibility in channel contact configuration. Multiple contacts can be programmed to activate simultaneously to ensure optimal stimulation. The implantation procedures in this laryngeal pacing system are more invasive than the MED-EL system, and battery replacement does require a skin incision to access the IPG. However, once implanted, electrode contact redundancy makes the system robust against electrode migration, alleviating the potential for multiple revision surgeries. Finally, this line of research is investigating bilateral stimulation of the PCA muscle, which is expected to produce even greater, near normal, ventilation outcomes compared with unilateral pacing. While unilateral laryngeal pacing outcomes suggest this intervention is an assistive device for breathing, the goal of bilateral laryngeal pacing is to completely rehabilitate a patient with BVFP, restoring them to a fully active lifestyle. This Infinity™ DBS system is currently being investigated in our ongoing research, including an FDA approved clinical trial of bilateral pacing (NCT03085316).
Conclusion
In the last few decades, research in FES for the reanimation of the PCA has successfully translated from animal models to human clinical trials for unilateral pacing and bilateral pacing. At this time, there are two primary approaches for laryngeal pacing in BVFP patients. Currently, unilateral pacing can be considered a breathing assist device and may not be appropriate for individuals who wish to engage in exercise. Mueller et al. have developed a minimally invasive unilateral pacing system which may be ideal for patients who would prefer less invasive implantation and are not concerned with cosmesis. Bilateral pacing can potentially restore ventilation to normal and allow strenuous exercise, as demonstrated in canines. Zealear et al. have developed a fully implantable bilateral pacing system with greater electrode redundancy and stability, resulting in a system that is robust against electrode migration or damage. Future studies should focus on systems that incorporate an inspiratory trigger for stimulation; however, animal and human studies report swallowing and/or voice were not impaired in the absence of inspiratory triggering.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Dedo HH. The paralyzed larynx: an electromyographic study in dogs and humans. Laryngoscope. 1970;80(10):1455–517. https://doi.org/10.1288/00005537-197010000-00001.
Holinger LD, Holinger PC, Holinger PH. Etiology of bilateral abductor vocal cord paralysis: a review of 389 cases. Ann Otol Rhinol Laryngol. 1976;85(4):428–36. https://doi.org/10.1177/000348947608500402.
Feehery JM, Pribitkin EA, Heffelfinger RN, Lacombe VG, Lee D, Lowry LD, et al. The evolving etiology of bilateral vocal fold immobility. J Voice. 2003;17(1):76–81. https://doi.org/10.1016/S0892-1997(03)00030-4.
• Li Y, Garrett G, Zealear D. Current treatment options for bilateral vocal fold paralysis: a state-of-the-art review. Clin Exp Otorhinolaryngol. 2017;10(3):203–12. https://doi.org/10.21053/ceo.2017.00199. This article presents an excellent overview of current and emerging interventions for BVFP.
Dedo HH. Electromyographic and visual evaluation of recurrent laryngeal nerve anastomosis in dogs. Ann Otol Rhinol Laryngol. 1971;80(5):664–8. https://doi.org/10.1177/000348947108000507.
Flint PW, Downs DH, Coltrera MD. Laryngeal synkinesis following reinnervation in the rat: neuroanatomic and physiologic study using retrograde fluorescent tracers and electromyography. Ann Otol Rhinol Laryngol. 1991;100(10):797–806. https://doi.org/10.1177/000348949110001003.
Siribodhi C, Sundmäker W, Atkins JP, Bonner FJ. Electromyographic studies of laryngeal paralysis and regeneration of laryngeal motor nerves in dogs. Laryngoscope. 1963;73(2):148–64. https://doi.org/10.1288/00005537-196302000-00002.
Tashiro T. Experimental studies on the reinnervation of larynx after accurate neurorrhaphy. Laryngoscope. 1972;82(2):225–36. https://doi.org/10.1288/00005537-197202000-00010.
Crumley RL, McCabe BF. Regeneration of the recurrent laryngeal nerve. Otolaryngol Neck Surg. 1982;90(4):442–7. https://doi.org/10.1177/019459988209000414.
Crumley RL. Laryngeal synkinesis: its significance to the laryngologist. Ann Otol Rhinol Laryngol. 1989;98(2):87–92. https://doi.org/10.1177/000348948909800201.
Seddon HJ. Three types of nerve injury. Brain. 1943;66(4):237–88. https://doi.org/10.1093/brain/66.4.237.
Zealear DL, Billante CR. Neurophysiology of vocal fold paralysis. Otolaryngol Clin N Am. 2004;37(1):1–23. https://doi.org/10.1016/S0030-6665(03)00165-8.
Zealear DL, Dedo HH. Control of paralysed axial muscles by electrical stimulation. Acta Otolaryngol. 1977;83:514–27. https://doi.org/10.3109/00016487709128880.
Dedo H. Studies of larynx and pharynx physiology. 1973. NIH grant application.
Obert PM, Young KA, Tobey DN. Use of direct posterior cricoarytenoid stimulation in laryngeal paralysis. Arch Otolaryngol. 1984;110(2):88–92. https://doi.org/10.1001/archotol.1984.00800280022007.
Bergmann K, Warzel H, Eckhardt H-U, Gerhardt H-J. Respiratory rhythmically regulated electrical stimulation of paralyzed laryngeal muscles. Laryngoscope. 1984;94(10):1376–80. https://doi.org/10.1288/00005537-198410000-00022.
Bergmann K, Gerhardt H-J, Warzel H, Eckhardt H-U, Hopstock U, Hermann V. Long-term implantation of a system of electrical stimulation of paralyzed laryngeal muscles in dogs. Laryngoscope. 1988;98(4):455–9. https://doi.org/10.1288/00005537-198804000-00020.
Sanders I. Electrical stimulation of laryngeal muscle. Otolaryngol Clin N Am. 1991;24(5):1253–74. https://doi.org/10.1016/S0030-6665(20)31079-3.
Zrunek M, Carraro U, Catani C, Scabolcs M, Gruber H, Streinzer W, et al. Functional electrostimulation of the denervated posticus muscle in an animal experiment: histo- and biochemical results. Laryngorhinootologie. 1986;65(11):621–7. https://doi.org/10.1055/s-2007-1008050.
Zealear DL, Rainey CL, Tanabe T, Jerles ML, Herzon GD. Technical approach for reanimation of the chronically denervated larynx by means of functional electrical stimulation. Ann Otol Rhinol Laryngol. 1994;103(9):705–12. https://doi.org/10.1177/000348949410300908.
Zealear DL, Billante CR, Chongkolwatana C, Rho YS, Hamdan A-L, Herzon GD. The effects of chronic electrical stimulation on laryngeal muscle physiology and histochemistry. ORL. 2000;62(2):81–6. https://doi.org/10.1159/000027722.
Mueller AH. Laryngeal pacing for bilateral vocal fold immobility. Curr Opin Otolaryngol Head Neck Surg. 2011;19(6):439–43. https://doi.org/10.1097/MOO.0b013e32834cb7ba.
Zealear DL, Rainey CL, Herzon GD, Netterville JL, Ossoff RH. Electrical pacing of the paralyzed human larynx. Ann Otol Rhinol Laryngol. 1996;105(9):689–93. https://doi.org/10.1177/000348949610500904.
Zealear DL, Billante CR, Courey MS, Netterville JL, Paniello RC, Sanders I, et al. Reanimation of the paralyzed human larynx with an implantable electrical stimulation device. Laryngoscope. 2003;113(7):1149–56. https://doi.org/10.1097/00005537-200307000-00010.
Billante CR, Courey MS, Zealear DL, Netterville JL. Effect of chronic electrical stimulation of laryngeal muscle on voice. Ann Otol Rhinol Laryngol. 2002;111(4):328–32. https://doi.org/10.1177/000348940211100408.
Li Y, Pearce EC, Mainthia R, Athavale SM, Dang J, Ashmead DH, et al. Comparison of ventilation and voice outcomes between unilateral laryngeal pacing and unilateral cordotomy for the treatment of bilateral vocal fold paralysis. ORL. 2013;75(2):68–73. https://doi.org/10.1159/000345501.
Förster G, Arnold D, Bischoff SJ, Schubert H, Scholle H-C, Müller AH. Laryngeal pacing in minipigs: in vivo test of a new minimal invasive transcricoidal electrode insertion method for functional electrical stimulation of the PCA. Eur Arch Otorhinolaryngol. 2013;270(1):225–31. https://doi.org/10.1007/s00405-012-2141-1.
Förster G, Arnold D, Bischoff S, et al. Pre-clinical evaluation of a minimally invasive laryngeal pacemaker system in mini-pig. Eur Arch Otorhinolaryngol. 2016;273(1):151–8. https://doi.org/10.1007/s00405-015-3735-1.
Mueller AH, Hagen R, Foerster G, Grossmann W, Baumbusch K, Pototschnig C. Laryngeal pacing via an implantable stimulator for the rehabilitation of subjects suffering from bilateral vocal fold paralysis: a prospective first-in-human study. Laryngoscope. 2016;126(8):1810–6. https://doi.org/10.1002/lary.25792.
• Mueller AH, Hagen R, Pototschnig C, et al. Laryngeal pacing for bilateral vocal fold paralysis: voice and respiratory aspects. Laryngoscope. 2017;127(8):1838–44. https://doi.org/10.1002/lary.26428. This study, in conjunction with Meuller et al., 2016, presents the respiratory outcomes of the first implantation of the MED-EL laryngeal pacing system in humans. Findings indicate this minimally-invasive system increases PIF and PEF without negatively affecting voice outcomes.
Zealear DL, Kunibe I, Nomura K, Billante C, Singh V, Huang S, et al. Rehabilitation of bilaterally paralyzed canine larynx with implantable stimulator. Laryngoscope. 2009;119(9):1737–44. https://doi.org/10.1002/lary.20587.
Katada A, Van Himbergen D, Kunibe I, et al. Evaluation of a deep brain stimulation electrode for laryngeal pacing. Ann Otol Rhinol Laryngol. 2008;117(8):621–9. https://doi.org/10.1177/000348940811700813.
Nomura K, Kunibe I, Katada A, Wright CT, Huang S, Choksi Y, et al. Bilateral motion restored to the paralyzed canine larynx with implantable stimulator. Laryngoscope. 2010;120(12):2399–409. https://doi.org/10.1002/lary.21065.
Zealear DL. Respiratory triggered, bilateral laryngeal stimulator to restore normal ventilation in vocal fold paralysis. Published online September 28, 2010. https://patents.google.com/patent/US7805195B2/en. Accessed 13 Aug 2019.
Funding
This study is supported by the NIH National Institute of Deafness and Communication Disorders clinical trial grant U01 DC016033.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
David L. Zealear reports two patents issued (US patent 7,805,195 and US patent 8,050,766).
The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maria E. Powell and David L. Zealear are cofirst authors.
This article is part of the Topical collection on Neurolaryngology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Powell, M.E., Zealear, D.L., Li, Y. et al. Unilateral and Bilateral Laryngeal Pacing for Bilateral Vocal Fold Paralysis. Curr Otorhinolaryngol Rep 8, 395–401 (2020). https://doi.org/10.1007/s40136-020-00313-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-020-00313-7