Abstract
Screening for nasopharyngeal carcinoma has been advocated in countries where the disease is endemic, especially among family members of nasopharyngeal carcinoma patients, as they are at significantly higher risk. Screening programmes are generally based on nasoendoscopy and Epstein–Barr virus (EBV) IgA serology tests, which have varying sensitivities and specificities. This article highlights the importance of screening for nasopharyngeal carcinoma and discusses current screening strategies and their outcomes. Challenges faced in identifying a good screening test are highlighted, and newer screening tools, including EBV DNA load and narrow-band imaging, are discussed. Recently developed molecular assays based on nasopharyngeal swabs are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is an uncommon cancer in most of the world, ranking as the 24th most frequently diagnosed cancer worldwide, with an incidence rate of 0.3–2.1 per 100,000 and accounting for only 0.7 % of the global cancer burden [1]. However, dramatically elevated rates are observed in certain populations. Of the geographical areas, NPC has the highest incident rate in Southeast Asia, as the 6th most common cancer among males (incidence rate 6.4 per 100,000) [1]. Populations with a high incidence include the Singapore Chinese, Malaysian Sarawak Bidayuh, and the Southern Chinese from Hong Kong and Guangdong. Incidence rates in these higher-risk populations range from 12.5 to 31.5 per 100,000 [2, 3]. Among Singapore males, it is the 7th most common cancer, with an incidence rate of 13.6 per 100,000 [4].
The distribution of NPC in particular geographic and ethnic groups suggests both environmental and genetic components in its etiology. Familial clustering of NPC has been widely reported and populations from high-risk areas continue to demonstrate a high risk of developing NPC even after migration to low-risk countries [5, 6]. Furthermore, first-degree relatives of NPC patients have a four-fold to ten-fold elevated risk of developing NPC, compared to those without a family history [2]. Certain genetic alleles, such as HLA-A2, B14, and B46, have also been associated with higher NPC risk [7]. Environmental risk factors with a strong association include intake of salted fish, and reduced intake of fresh fruits and vegetables.
Another important component in the etiology of NPC is the Epstein–Barr virus (EBV), a ubiquitous herpesvirus carried in a latent, non-pathogenic state by 80–90 % of all humans [8]. The association between EBV antibodies and NPC, which forms the basis for the majority of screening tests today, has been established since the late 1960s, with correlation of titres to the stage of disease and the amount of tumour present [9, 10].
The WHO classifies NPC into two histologic groups, keratinizing squamous cell carcinoma (type I) and non-keratinising carcinoma (type 2), further subdivided into differentiated (type 2a) and undifferentiated (type 2b) tumors. In endemic countries, the vast majority of NPC is of the type 2 variety, which has a strong association with EBV infection. Population-based screening studies have thus only been performed in endemic countries to date, with different types of EBV serology and viral DNA load tests used as screening tools.
Role for Screening
Although NPC constitutes an important health problem in endemic countries, its incidence in the general population remains low compared to other major cancers. In some developed countries, its incidence is decreasing, possibly due to lifestyle modifications and a decreased intake of preserved foods [11, 12]. Screening for NPC is challenging, as there is no clear pre-malignant phase, unlike other cancers such as the adenoma-carcinoma sequence in colorectal cancer, or the spectrum of pre-malignant conditions (varying degrees of dysplasia to carcinoma-in situ) in cervical cancer. Nonetheless, early detection of NPC reduces the morbidity of treatment, as early stage disease is treated with radiation therapy alone, compared to advanced disease, which is treated with chemotherapy and radiation therapy. Furthermore, NPC patients are often middle-aged and economically active; detection at an early stage significantly improves survival and may be economically advantageous in a country with endemic disease.
Although the majority of NPC patients have a visible mass in the nasopharynx on nasoendoscopy, the occasional patient may present with a normal-appearing nasopharynx. To the clinician’s relief, these patients will usually have palpable cervical lymph node metastasis [13]. With regards to laboratory methods, no single blood test has managed to achieve sufficiently high sensitivity and specificity to allow for a single test to be used for screening in the general population. An inadequately sensitive test would give a high false negative rate, missing patients with NPC. Conversely, inadequate specificity would result in too many false positives, and would result in unnecessary nasoendoscopies, nasopharynx biopsies and follow-up visits. Although screening the general population appears to be problematic, screening programmes for high-risk family members have been proposed due to higher incidence rates among family members.
Screening high-risk family members for NPC in endemic populations with serology and nasoendoscopy has demonstrated high incidence rates of 77–266/100,000 person-years, which is much higher than the general population [14, 15]. In the strongest evidence yet, a prospective screening study by Ng et al. [14] demonstrated that screening family members aids the detection of NPC at early stages and improves disease-free survival. Seventeen cases of NPC were identified out of 1,199 asymptomatic family members, and 59 % presented at early stage (I and II) compared to only 24 % of symptomatic NPC patients presenting at an early stage during the same period. NPC cases picked up on screening also had significantly higher disease-free survival with a hazard ratio of 0.319 for recurrence or death relative to symptomatic NPC patients (p = 0.04).
Using Markov chain models, Choi et al. [16] analysed the efficacy of screening strategies for familial NPC in the same cohort. The mean sojourn time (duration between asymptomatic and clinical phase of disease) was estimated to be 3.12 years, consistent with previous estimates by Ji et al. [17]. A long sojourn time is important for successful disease screening. Based on simulation models, Choi et al. showed that stratifying family members based on EBV status could significantly improve the efficacy of screening programmes. By selecting EBV-positive individuals for annual screening while screening EBV-negative individuals triennially, the number of screening visits could be reduced by 40 %, with a reduction in disease pick-up by less than 1 %. Using serology results to stratify patients based on their risk, and tailoring a risk-specific follow-up screening plan would help maximise the use of resources in a screening programme.
Apart from screening of high-risk family members, general population-based screening studies have also been carried out with serology tests and reviews of cancer registries, with lower incidence rates reported compared to screening of high-risk family members [18, 19•]. A summary of the results of these studies can be found in Table 1.
EBV Serology Tests
EBV serology has been the main test used in screening. This is based on the observation that NPC patients have elevated IgG and IgA antibody titres to the EBV viral capsid antigen (VCA) and EBV early antigen (Ea), as well as increased IgG against the latent viral nuclear antigens 1 and 2 (EBNA-1, EBNA-2) and neutralizing antibodies against EBV-specific DNase [2]. EBV IgA antibodies especially may be elevated in the years before diagnosis (sometimes up to 10 years before diagnosis), and elevated titres have been shown to increase the risk of NPC [17, 20].
Chien et al. [20] demonstrated in a cohort of 8,891 Taiwanese men that elevated EBV VCA IgA antibodies resulted in a relative risk of 22.0 compared to negative individuals, and a cummulative risk of 301.3 per 100,000 person-years. The result was less pronounced for EBV Anti-EBV DNase antibodies, with a relative risk of 3.5 and a cummulative risk of 45.7 per 100,000 person-years. The authors also found that EBV IgA levels were elevated before diagnosis, and the relative risk of developing NPC with a positive EBV VCA IgA titre was especially high in the first 5 years of follow-up, at 55.5 compared to EBV VCA IgA negative individuals. However, only 1.2 % of the study population had positive EBV VCA IgA titres, while other publications report higher positive rates in the general population [19•, 21–23]. Another large cohort study involving 18,986 Chinese subjects similarly found that EBV VCA IgA titres correlated with the risk of developing NPC. Moreover, individuals who had higher titres for EBV VCA IgA (≥1:20) and a positive EBV Ea IgA serology had the most pronounced risk, with a hazard ratio of 88.7 [19•].
In a study comparing antibody titers against EBV VCA IgA, EBV nuclear antigen-1 (EBNA-1) IgA, and DNase among 2,444 unaffected relatives of NPC cases in Taiwan, individuals with positive EBNA-1 IgA titers had a 4.7 times relative risk compared to those who were negative. Its sensitivity appeared to be limited, as it identified only 50 % (7 of 14) NPC cases in the study, but it gave the best sensitivity among the three markers tested [24].
While it is clear that EBV serology tests are helpful in stratifying patients based on risks, the choice serology test to be used for screening is still a matter of debate. Although EBV VCA IgA gives a very good sensitivity, it has a relatively poor specificity and an unacceptably high false positive rate, of up to 53.2 % in controls [22]. Its lower specificity limits its role as a screening tool, as it would identify too many false positive individuals, requiring further clinical evaluation and follow-up. EBV Ea IgA, on the other hand, appears to give a very high specificity of 95–100 % and relatively poorer sensitivity of between 72 and 79 % [23, 25–27].
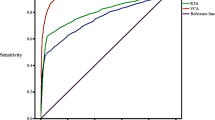
Chang et al. [28] compared EBV VCA IgA and a combined EBV Ea + EBNA-1 IgA test in 156 NPC patients and 264 controls. Based on receiver operating characteristic (ROC) curves, EBV EA + EBNA-1 IgA (AUC: 0.952) proved superior to EBV VCA IgA (AUC: 0.888) as a screening test. A summary of the sensitivity and specificities of various EBV serology tests can be found in Table 2.
EBV DNA Load
The EBV DNA load test is a real-time quantitative PCR targeting the BamHI-W and EBNA-1 regions of the EBV genome in plasma or serum samples. The test, first designed in 1999, demonstrated a distinction in EBV DNA load between NPC patients and controls, with 96 % of NPC patients having detectable EBV DNA compared to 7 % of controls [29]. However, subsequent studies have reported lower detection rates of 53–74 % among NPC patients, and also demonstrated limited sensitivity for this test [30, 31]. Chan et al. [25] evaluated EBV DNA load in 218 consecutive serum samples, of which 51 had a diagnosis of NPC, and demonstrated a sensitivity of 56.4 %, specificity of 98.2 %, positive predictive value of 91.2 % and negative predictive value of 87 %.
A comparison of 160 untreated NPC patients with 76 healthy donors with EBV DNA load also showed a limited sensitivity of 68.8 % and a specificity of 88.2 %. Furthermore, the sensitivity and specificity results for EBV VCA IgA, EBV Ea IgA and EBV DNase antibody were superior compared to EBV DNA load in this study [27]. In another direct comparison of EBV DNA load and a combined EBV Ea + EBNA-1 IgA test, Chang demonstrated that EBV DNA load was poorer as a screening tool based on ROC curves (AUC: 0.893 vs. 0.952 respectively) [28].
Among high-risk family members, there are limited studies evaluating EBV DNA load as a screening tool. Depending on the assay used, the detection rate of EBV DNA among healthy family members ranged from 0 to 15 % [30, 31]. The current data in the literature suggests a limited role for EBV DNA load as a screening tool for NPC.
Narrow-Band Imaging
Narrow-band imaging (NBI) is a recently described technique used in addition to traditional nasoendoscopy with white-light imaging. Using narrow-band optical filters selecting blue and green light at wavelengths of 415 and 540 nm, epithelial and subepithelial microvascular patterns in the nasopharynx can be visualised. Lesions exhibiting a well demarcated brownish area or irregular, distorted microvascular patterns are suspicious for malignancy. With this additional imaging tool, superficial or submucosal lesions that may be missed by traditional white-light imaging can be identified, improving the detection rate of NPC on nasoendoscopy.
In a study evaluating 211 consecutive patients with nasopharyngeal lesions, Wen et al. [32] reported a significantly higher sensitivity of detecting NPC with NBI compared to white-light imaging (93.9 vs. 71.2 %). Another study evaluating 1,854 patients with both NBI and white-light imaging also found that NBI had a superior sensitivity (100 vs. 90.3 %) and specificity (99.2 vs. 75.4 %) compared to white-light imaging in identifying NPC [33].
Although NBI is helpful in identifying malignant nasopharyngeal lesions more accurately, its benefit over white-light imaging in screening programmes still remains to be established. Ho et al. [34] screened 211 family members of NPC patients with NBI and white-light imaging, and found good correlation between both techniques. All four family members diagnosed with NPC in this study had suspicious findings on both NBI and white-light imaging. In screening programmes for high-risk family members in endemic regions, the threshold to biopsy any suspicious nasopharynx lesions would be low and NBI may not be helpful in improving specificity. However, the occasional non-exophytic lesion which may be missed on white-light imaging may be picked up on NBI, thus improving sensitivity.
Other Screening Tools
Molecular assays based on nasopharyngeal swabs have also been developed as possible screening tools. The assays are based on polymerase chain reaction (PCR) techniques to detect EBV-related antigens such as latent membrane protein-1 gene (LMP-1) and Epstein–Barr nuclear antigen gene (EBNA) [35]. Studies detecting the methylation status of tumor suppressor genes such as p15, p16, Ras association domain family 1 (RASSF1A), death-associated protein kinase (DAPK) and E-cadherin have also demonstrated epigenetic changes detectable in mouth and throat fluids and nasopharyngeal swabs [36].
Recently, Zhang et al. [37•] developed a multiplex PCR reaction, co-amplifying EBNA-1, LMP-1, methylated RASSF1A, and DAPK in a single reaction. Among the 69 samples tested (49 NPC patients and 20 normal controls), the sensitivity of detecting NPC from nasopharyngeal swabs was 98 %, with the specificity as high as 100 %. As nasopharyngeal swabs are non-invasive, this method of testing is likely to be acceptable to most of the population. Furthermore, the combination of four markers in a single PCR reaction provides for cost-savings, especially if PCR reactions are run in bulk. However, unlike serology, which can be performed along with other screening blood tests for diabetes or hyperlipidemia, nasopharyngeal swabs will require a separate procedure, which may reduce its take-up rate. Further evaluation of nasopharyngeal swab molecular assays in screening studies should be considered to test its utility as a screening tool.
Current Screening Recommendations
Recommendations have been made for screening family members of NPC patients in countries where NPC is endemic. In Hong Kong, screening of family members above the age of 30 with nasoendoscopy and EBV-specific IgA ELISA [38] is recommended. Individuals with positive ELISA results are further evaluated with EBV VCA IgA and nasopharynx biopsies. If these tests are negative, yearly follow-up is recommended. In cases where EBV VCA IgA is positive and nasopharynx biopsies negative, further deep nasopharynx biopsies are recommended.
In Singapore, annual screening with EBV serology and nasoendoscopy is recommended in persons with two or more family members with NPC [39]. The serology tests of choice performed are EBV VCA IgA, with a high sensitivity, and EBV Ea IgA, with a high specificity. Titres of 1:160 for EBV VCA IgA and 1:5 for EBV Ea IgA are considered significant and would warrant further investigation with nasopharynx biopsies and MRI scans.
Future Directions
One of the key challenges in NPC screening is the development of a minimally invasive screening test with good sensitivity and specificity. However, based on the sensitivity and specificity results described above, EBV Ea IgA appears to give the best balance, with its superiority as a screening test further confirmed by ROC curve. Furthermore, its high specificity means a low rate of false positive results, which is crucial in screening a large population with a low prevalence of disease.
It would be interesting to see the results of prospective screening studies with both nasoendoscopy and serology tests. Even though EBV VCA IgA and EBV DNase antibodies have been demonstrated to be elevated in the years preceeding NPC diagnosis, the time taken for tumor to develop and the true asymptomatic phase of the disease after seroconversion is unknown due to a lack of evaluation of the nasopharynx in currently published studies. Nasoendoscopy findings will help establish more accurately the temporal sequence between seroconversion and subsequent development of the disease.
Prospective screening studies evaluating multiple screening tests will also allow for better comparison of sensitivities and specificities and the calculation of positive and negative predictive values, which are lacking in current comparison studies in the general population. Cost-analysis studies of screening programmes in the general population and high risk family members should also be undertaken, considering different permutations of screening tests and frequency of clinical consultations based on risk stratification.
The recently reported nasopharyngeal swab molecular assays hold promise, and their sensitivity and specificity may be studied alongside serology tests in a screening programme. The non-invasive nature of the test, and the high sensitivity and specificity reported, suggests its potential as a screening tool.
Conclusion
Nasopharyngeal carcinoma has high incidence rates in certain populations, and screening programmes in these populations, especially among high risk family members, will be beneficial for survival, as patients with early stage disease have a much better prognosis. The development of a cost-effective and resource-efficient screening model should involve the use of EBV serology titres for risk-stratification. In order for screening to be extended to the general population, screening tests with better sensitivity and specificity will need to be developed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomark Prev. 2006;15(10):1765–77.
Devi BC, Pisani P, Tang TS, Parkin DM. High incidence of nasopharyngeal carcinoma in native people of Sarawak, Borneo Island. Cancer Epidemiol Biomark Prev. 2004;13(3):482–6.
Lee HP, et al. Trends in cancer incidence in Singapore 2004–2008. Singapore : Singapore Cancer Registry Interim Report, Health Promotion Board; 2010.
Parkin DM, Iscovich J. Risk of cancer in migrants and their descendants in Israel: carcinomas and germ-cell tumours. Int J Cancer. 1997;70:654–60.
Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer incidence in five continents, vol. VIII. Lyon: IARC scientific publications; 2002.
Goldsmith DB, West TM, Morton R. HLA associations with nasopharyngeal carcinoma in Southern Chinese: a meta-analysis. Clin Otolaryngol. 2002;27:61–7.
Klein G. Epstein–Barr virus (EBV) in encyclopedia of genetics. Philadelphia: Elsevier; 2001. p. 641–4.
De Schryver A, Friberg S, Klein G, Henle W, Henle G, et al. Epstein–Barr virus-associated antibody patterns in carcinoma of the post-nasal space. Clin Exper Immunol. 1969;5(5):443.
De Schryver A, Klein G, Henle W, Henle G. EB virus-associated antibodies in caucasian patients with carcinoma OE the nasopharynx and in long-term survivors after treatment. Int J Cancer. 1974;13(3):319–25.
Hsu C, Shen Y-C, Cheng C–C, Hong R-L, Chang C-J, Cheng A-L. Difference in the incidence trend of nasopharyngeal and oropharyngeal carcinomas in Taiwan: implication from age-period-cohort analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:856–61.
Seow A, Koh WP, Chia KS, Shi LM, Lee HP, Shanmugaratnam K. Cancer incidence in Singapore 1968–2002. Singapore Cancer Registry Report No. 6;2004.
Loh KS, Petersson F. Nonexophytic nasopharyngeal carcinoma: high frequency of advanced lymph node and distant metastasis. Otolaryngol Head Neck Surg. 2011;145(4):594–8.
Ng WT, Choi CW, Lee MCH, Law LY, Yau TK, Lee AWM. Outcomes of nasopharyngeal carcinoma screening for high risk family members in Hong Kong. Fam Cancer. 2009;9(2):221–8.
Yu KJ, Hsu W-L, Pfeiffer RM, et al. Prognostic utility of Anti-EBV antibody testing for defining NPC risk among individuals from high-risk NPC families. Clin Cancer Res. 2011;17(7):1906–14.
Choi CW, Lee MCH, Ng WT, Law LY, Yau TK, Lee AWM. An analysis of the efficacy of serial screening for familial nasopharyngeal carcinoma based on Markov chain models. Fam Cancer. 2010;10(1):133–9.
Ji MF, Wang DK, Yu YL, et al. Sustained elevation of Epstein–Barr virus antibody levels preceding clinical onset of nasopharyngeal carcinoma. Br J Cancer. 2007;96(4):623–30.
Hsu W-L, Yu KJ, Chien Y-C, et al. Familial tendency and risk of nasopharyngeal carcinoma in Taiwan: effects of covariates on risk. Am J Epidemiol. 2010;173(3):292–9.
• Cao S-M, Liu Z, Jia W-H, et al. Fluctuations of Epstein–Barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. Najbauer J, ed. PLoS ONE. 2011;6(4):e19100. The authors describe a population-based screening program in China involving a total of 18,986 subjects. The study demonstrated significantly higher risks for NPC with positive serology titres for EBV VCA IgA and EBV Ea IgA.
Chien YC, Chen JY, Liu MY, et al. Serologic markers of Epstein–Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345(26):1877–82.
O TM, Yu G, Hu K, et al. Plasma Epstein–Barr virus immunoglobulin A and DNA for nasopharyngeal carcinoma screening in the United States. Otolaryngol Head Neck Surg. 2007;136(6):992–7.
Low WK, Leong JL, Goh YH, Fong KW. Diagnostic value of Epstein–Barr viral serology in nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2000;123(4):505–7.
Chan SH, Soo MY, Gan YY, Fones-Tan A, Sim PS, Kaur A, Chew CT. Epstein Barr virus (EBV) antibodies in the diagnosis of NPC–comparison between IFA and two commercial ELISA kits. Singapore Med J. 1998;39(6):263–5.
Yu KJ, Hsu W-L, Pfeiffer RM, et al. Prognostic utility of anti-EBV antibody testing for defining NPC risk among individuals from high-risk NPC families. Clin Cancer Res. 2011;17(7):1906–14.
Chan KH, Gu YL, Ng F, et al. EBV specific antibody-based and DNA-based assays in serologic diagnosis of nasopharyngeal carcinoma. Int J Cancer. 2003;105(5):706–9.
Wong MM, Lye MS, Cheng HM, Sam CK. Epstein–Barr virus serology in the diagnosis of nasopharyngeal carcinoma. Asian Pac J Allergy Immunol. 2005;23(1):65–7.
Luo YL, Ou GP, Chi PD, Liang YN, Liu YH, Huang MY. Combined determination of Epstein–Barr virus-related antibodies and antigens for diagnosis of nasopharyngeal carcinoma. Chin J Cancer. 2009;28(1):76–8.
Chang KP, Hsu CL, Chang YL, et al. Complementary serum test of antibodies to Epstein–Barr virus nuclear antigen-1 and early antigen: a possible alternative for primary screening of nasopharyngeal carcinoma. Oral Oncol. 2008;44(8):784–92.
Lo YM, Chan L, Lo KW, et al. Quantitative analysis of cell-free Epstein–Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999;59(6):1188.
Yang XR, Goldstein AM, Chen CJ, et al. Distribution of Epstein–Barr viral load in serum of individuals from nasopharyngeal carcinoma high-risk families in Taiwan. Int J Cancer. 2006;118(3):780–4.
Baizig NM, Morand P, Seigneurin JM, et al. Complementary determination of Epstein–Barr virus DNA load and serum markers for nasopharyngeal carcinoma screening and early detection in individuals at risk in Tunisia. Eur Arch Otorhinolaryngol. 2011;269(3):1005–11.
Wen Y-H, Zhu X-L, Lei W-B, Zeng Y-H, Sun Y-Q, Wen W-P. Narrow-band imaging: a novel screening tool for early nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2012;138(2):183–8.
Yang H, Zheng Y, Chen Q, Xiong H, Chen B, Zhang Z, Huang X, Peng J. The diagnostic value of narrow-band imaging for the detection of nasopharyngeal carcinoma. ORL J Otorhinolaryngol Relat Spec. 2012;74(5):235–9.
Ho CY, Chan KT, Chu PY. Comparison of narrow-band imaging and conventional nasopharyngoscopy for the screening of unaffected members of families with nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2013;270(9):2515–20.
Hao SP, Tsang NM, Chang KP, Ueng SH. Molecular diagnosis of nasopharyngeal carcinoma: detecting LMP-1 and EBNA by nasopharyngeal swab. Otolaryngol Head Neck Surg. 2004;131(5):651–4.
Chang HW, Chan A, Kwong DL, Wei WI, Sham JS, Yuen AP. Evaluation of hypermethylated tumor suppressor genes as tumor markers in mouth and throat rinsing fluid, nasopharyngeal swab and peripheral blood of nasopharygeal carcinoma patient. Int J Cancer. 2003;105(6):851–5.
• Zhang Z, Sun D, Hutajulu SH, Nawaz I, Nguyen Van D, Huang G, Haryana SM, Middeldorp JM, Ernberg I, Hu LF. Development of a non-invasive method, multiplex methylation specific PCR (MMSP), for early diagnosis of nasopharyngeal carcinoma. PLoS One. 2012;7(11):e45908. The authors describe a multiplex methylation specific-PCR assay designed to detect tumor-specific methylation status of several NPC-related genes through a single PCR reaction with tumor DNA derived from nasopharyngeal swabs.
Ng WT, Ying ACH. Guidelines on cancer prevention, early detection and screening nasopharyngeal carcinoma. The Hong Kong Anti-Cancer Society, Hong Kong. 2008. http://www.hkacs.org.hk/uploadimages/download/00147/Guideline%20on%20NPC%20prevention,%20detection%20&%20screening%20%28full%20recommendation%29.pdf. Accessed 11 Nov 2013.
Lee HP et al. Ministry of Health Clinical Practice Guidelines 1/2010—cancer screening. Ministry of Health, Singapore. 2010. http://www.moh.gov.sg/content/moh_web/healthprofessionalsportal/doctors/guidelines/cpg_medical.html. Accessed 11 Nov 2013.
Compliance with Ethics Guidelines
Conflict of Interest
Joshua K Tay, Ming Yann Lim, and Jeeve Kanagalingam declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tay, J.K., Lim, M.Y. & Kanagalingam, J. Screening in Nasopharyngeal Carcinoma: Current Strategies and Future Directions. Curr Otorhinolaryngol Rep 2, 1–7 (2014). https://doi.org/10.1007/s40136-013-0035-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-013-0035-4