Abstract
Transcranial magnetic resonance imaging guided focused ultrasound surgery (tcMRIgFUS) has recently taken root as a novel, noninvasive alternative to traditional neurosurgery. The ability to treat a variety of neurological diseases by precisely focusing ultrasound energy to a desired target deep inside the brain while leaving collateral structures unaltered is certainly attractive. Ongoing preclinical and phase I clinical nonrandomized studies have demonstrated that ultrasound energy can be focused through the intact skull, without overheating it, that the treatment location can be visualized, and that induced temperature changes can be monitored in nearly real time. Varying the ultrasound parameters not only allows ablation of pathological tissue, such as brain tumors and metastases, or silencing of dysfunctional neuronal circuits, but also opens up the blood–brain barrier for targeted drug delivery and modulation of neural function. Here, key developments, the current status, and the potential of tcMRIgFUS for neurological applications are presented, and important issues for ongoing research are delineated.
Similar content being viewed by others
Introduction
Despite modern technologies, neurosurgical resection still bears a risk of damaging collateral healthy brain tissue. As a consequence, for more than a century there has been a continuous search for minimally invasive neurosurgical interventions that promise new ways for the management of a variety of brain diseases [1]. Refined techniques have been developed over the years to improve accurate targeting while avoiding undesirable side effects [2]. One of the least invasive methods is stereotactic neurosurgery, which uses a stereotactic frame for delivering radiation, targeting of electrodes, and focal resection of brain tissue to treat tumors, movement disorders, pain syndromes, epilepsy, and vascular malformations [3, 4]. A better understanding of the pathophysiological mechanisms and thorough knowledge of the neurotransmitters involved in functional brain disorders have led to increased acceptance of stereotactic interventions.
In this context, the ability to treat deeply seated brain tissue by precisely focusing ultrasound energy to a desired target while leaving collateral structures unaltered is certainly attractive [5]. Magnetic resonance imaging (MRI)-guided focused ultrasound surgery (MRIgFUS) has taken root as a novel, noninvasive thermal ablation method that is image-guided and does not involve the potential serious late effects of ionizing radiation [6]. MRIgFUS integrates the focusing capability of ultrasound energy with MRI for target definition, treatment planning, and intervention guidance with high precision while simultaneously monitoring the energy deposition in the tissue and the resulting temperature evolution in real time.
Transcranial MRIgFUS (tcMRIgFUS) is especially appealing, since brain disorders are a highly crucial treatment area that requires extremely precise intervention techniques. While delivering ablative energy noninvasively through the intact skull with high precision, yet leaving collateral brain structures unaltered, tcMRIgFUS has the potential to drive completely new treatment approaches for a variety of neurological and neuropsychiatric diseases to improve the shortcomings of established treatment strategies in the field. Although clinical studies have only just begun, tcMRIgFUS will eventually change the future treatment of many patients. In addition to the disruptive nature of thermal ablation by high-energy focused ultrasound (HIFU), future applications may also involve low-energy focused ultrasound (LIFU) for neuromodulation and for induction of temporal changes in the local permeability of the blood–brain barrier (BBB) and cell membranes for targeted drug delivery.
In this article, we describe the key features of tcMRIgFUS as a novel, noninvasive treatment modality for a variety of neurological disorders on the basis of preclinical and ongoing collaborative phase I clinical trials.
History of Application of Focused Ultrasound to the Brain
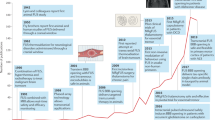
Modulation of pathological networks for treating functional brain disorders has a long history (Fig. 1), yet the precise mechanism of action and the optimal targets for treating the diverse motor, somatosensory, and emotional disturbances are still debated.
The concept of applying ultrasound energy to the brain for therapeutic neurosurgical interventions goes back to the seminal work of Lynn et al. [7, 8]. In carefully designed animal experiments they produced focal lesions deep inside the brain and spinal cord without damaging collateral nontargeted tissue using HIFU, which led to well-defined tissue modifications. The resulting reversible and irreversible specific behavioral changes and neurological dysfunctions made focused ultrasound a tool of considerable power for investigating basic brain mechanisms in neuroanatomical, behavioral, and physiological studies [9]. However, while initially trying to sonicate brain through the intact skull, they invariably damaged the skin and underlying tissue by producing burns to the scalp, muscles, and even meninges, which resulted from extremely strong attenuation of the ultrasound beam while passing through the skull bone. As a consequence, further preclinical and early clinical studies required craniectomies in order to avoid ultrasound attenuation, beam distortion, and beam reflection [10–13]. With this open skull technique it became possible to coagulate well-defined tissue volumes in the brain selectively and precisely by heat-induced protein denaturation without damaging the surrounding structures. Brain tumors, hyperkinetic and hypertonic movement disorders, epilepsy, intractable pain, violent behavior, and even neuropsychiatric disorders have been treated with good clinical results for almost 50 years [14–17]. However, apart from the necessity to remove parts of the skull bone, other technical limitations became apparent. The limited imaging possibility led to imprecise targeting with subsequent appreciable side effects and complications, especially when aiming at targets in the thalamus and basal ganglia for treating functional disorders. Although continuous improvements in transducer technology resulted in better targeting, the combination of rigid head fixation and the use of skull X-ray with direct ventriculography to obtain landmarks allowed stereotactic functional neurosurgery with astonishingly high precision [1]. Although the Fry brothers demonstrated the technical feasibility of transskull transmission of HIFU in the late 1970s, more than 20 years of intensive methodological and experimental research was necessary until noninvasive brain interventions became clinically feasible [18–20, 21••]. The development of multichannel high-power phased-array ultrasound transducers together with the implementation of computer programs to correct for beam aberration and the construction of MRI-compatible transducers allowing image guidance and real-time temperature monitoring were necessary to start clinical studies in selected neurosurgical patients [22••, 23••, 24•].
Today, more than 70 neurosurgical patient treatments in the context of worldwide coordinated clinical phase I studies have demonstrated that noninvasive tcMRIgFUS interventions can be safely performed without excessive heating of the scalp, skull, or brain surface or even damaging nontargeted brain tissue.
Intervention Procedure and Patient Treatment
TcMRIgFUS is at the core of a variety of new image-guided treatment strategies that are noninvasive, do not involve ionizing radiation, and can be applied repeatedly. Since it does not require full anesthesia and patients are awake during the whole intervention procedure, tcMRIgFUS can mostly be applied on an outpatient basis. However, it exploits complex thermal and nonthermal effects of ultrasound–tissue interaction to effect physiological changes in biological systems (Fig. 2).
The thermal effects produced by HIFU are mainly used for tissue ablation and mild hyperthermia for radiosensitization. Continuous pressure waves of HIFU are absorbed at the focus of the sonication, where mechanical energy is converted into thermal energy, heating up targeted tissue to a degree that induces irreversible protein denaturation and tissue necrosis [25]. Depending on the pressure amplitude and tissue characteristics, cavitation, i.e., the formation of gaseous cavities, can be induced especially at tissue interfaces. During stable cavitation, intact gas bubbles oscillate, leading to local mechanical effects, whereas during inertial cavitation, the violently oscillating bubbles collapse, causing high energy deposition with heat dissipation and tissue rupture [26].
The nonthermal predominantly mechanical effects of focused ultrasound surgery (FUS) are induced by LIFU. Theses effects arise by direct impaction of oscillating bubbles on cell membranes and tight junctions of the brain capillary endothelium, and include cavitation, radiation force, acoustic streaming, and other physical effects that are applied mostly to reversibly modulate physiological properties of biologic tissues, e.g., for BBB opening, acoustic neuromodulation, sonoporation of cell membranes, or reopening of occluded vessels, i.e., sonothrombolysis. To avoid inertial cavitation, ultrashort LIFU bursts are combined with the administration of preformed microbubbles, which are commonly used as an ultrasound contrast agent.
Intervention guidance and monitoring are provided by MRI and magnetic resonance thermometry (MRT) almost in real time. MRI offers excellent soft tissue contrast for intervention planning and safety assessment before effective sonication treatment, whereas MRT provides important information for intervention monitoring and outcome prediction. Posttreatment lesion assessment is done immediately after the treatment intervention by T2-weighted and contrast-enhanced T1-weighted MRI.
The tcMRIgFUS system (ExAblate 4000, InSightec LTD) is integrated into a clinical 3-T magnetic resonance scanner and consists of a half-spherical ultrasound transducer, radio-frequency driving electronics, a water cooling and degassing system, electronic interfaces to remotely control the magnetic resonance scanner and access the scanner’s image database, and a workstation next to the scanner console to operate and control the focused ultrasound procedure [27, 28] (Fig. 3a). On the basis of CT analysis and modeling of ultrasound beam aberration by the anisotropic skull bone, the phase and amplitude are set for each transducer element individually, allowing electronic steering of the focus [23••]. A stereotactic frame immobilizes the patient’s head and a water interface between the transducer and the patient’s head cools the skull bone and serves as acoustic coupling. MRI provides image guidance and real-time temperature monitoring during the entire ablation process by proton resonance frequency shift based MRT [29, 30].
The treatment session begins with fixating an MRI-compatible stereotactic frame (Radionics, Burlington, MA, USA) to the patient’s head (see Fig. 3b). The transducer is filled with degassed water, and a flexible silicon membrane is fitted around the patient’s head, sealing the space between the transducer and the scalp. For precise localization of the stereotactic target, a multiarchitectonic atlas of the human thalamus and basal ganglia [31] is used, and the spatial configuration of sonications needed to optimally cover the target volume is prescribed. Calibration and monitoring of the intervention procedure is comparable to that for extracranial MRIgFUS interventions and is performed as a two-step procedure: geometric verification of location of the acoustic hotspot, and dose verification to find the optimal acoustic power level and sonication duration. The sonication parameters for shaping the desired ablation volume are iteratively determined in a series of sonications with stepwise-increased acoustic power from 300 to 1,200 W with sonications of 12–25-s duration. Typically, six to nine such dose-verification sonications are applied and monitored by MRT in each session until an ablative peak temperature between 56 and 62 °C is reached.
a The patient’s head in the half-spherical multielement phased-array transducer, which is placed inside the magnetic resonance scanner. b The ExAblate 4000 transcranial magnetic resonance imaging (MRI)-guided focused ultrasound surgery (tcMRIgFUS) system (InSightec, Tirat-Carmel, Israel), with which the brain interventions are performed. The 30-cm-diameter hemispheric, 1,024-element phased-array ultrasound transducer is attached to a four-axis positioner mounted on a modified GE Healthcare patient table. The RF electronics drive the transducer at 650 kHz, with a maximum total acoustic power of 2,000 W
The size of the natural focus of the 650-kHz transducer is 3 mm × 3 mm × 4 mm. The acoustic absorption coefficient of tissue in different brain structures is considered uniform unless the tissue is calcified or scared. Increasing acoustic power will elevate the focal peak temperature, whereas increasing sonication duration will favor heat dissipation and tends to enlarge the lesion volume. There is no trajectory restriction. TcMRIgFUS allows any desired lesion volume to be shaped by electronically repositioning the thermal hotspot. During the cooling periods of several minutes between each sonication treatment, magnetic resonance images are acquired that typically show ablative lesions immediately. Moreover, since the patients are fully awake during the entire intervention procedure, the cooling periods are used for interviews and neurological examinations. Special attention is paid to vestibular and vegetative symptoms, which are regularly observed. They are interpreted as ultrasound-mediated neurostimulatory effects and are appreciated as valuable neurophysiological feedback information from the treated target volume. In addition, changes in somatosensory or even motor manifestations experienced during the course of the intervention procedure are recorded and carefully evaluated for positive therapeutic or negative adverse signs.
Current Applications
Functional Brain Disorders
In 2008 a clinical feasibility and safety study was started at the University of Zurich, and later in Solothurn, Switzerland, on patients with a history of chronic pain syndromes related to limb amputation, nerve root compression, “failed back surgery syndrome,” trigeminal neuralgia, nerve trauma, spinal cord lesion with paraplegia and tetraplegia, striatal lesion, brachial plexus avulsion, and thalamic infarcts using the 650-kHz ExAblate 4000 tcMRIgFUS system (InSightec, Tirat-Carmel, Israel) [22••, 24•]. The tcMRIgFUS intervention consists of one to three adjacent thermal ablations in the posterior part of the central lateral thalamic nucleus, contralateral to the origin of the predominant pain. In selected patients with severe and extended pain affecting bilateral extremities, hemibody pain syndromes, or serious trigeminal neuropathy, a bilateral central lateral thalamotomy is performed. On average, a peak temperature between 56 and 59 °C (range 54–64 °C) creates precisely located single thermal coagulation necrosis of 3–5 mm in diameter, depending on the sonication parameters used, with a targeting accuracy of less than 1 mm, when compared with the planned target coordinates. Interestingly, the size of the lesion shrinks over time to about 50 % of its original size shortly after the operation; it never enlarges like in other stereotactic interventions.
Postoperative evaluation with MRI immediately after the intervention reveals well-demarcated lesions with a small perifocal vasogenic edema of 2–3 mm (Fig. 4).
During the operation patients often experience somatosensory effects, e.g., disappearance of numbness or allodynia and appearance of normal sensation in the affected limb, and vestibular manifestations, such as dizziness or vertigo, because of ultrasound-based neuronal activation and/or transient therapeutic effects.
To date, more than 50 patients have been treated with good success (mean pain relief at 1 year follow-up between 55 and 60 %). A transient hemineglect with dysarthria is the only serious adverse effect experienced so far. It required neurorehabilitation [24•].
Currently patients with refractory essential tremor and Parkinson’s disease are treated at the University of Virginia (Charlottesville, USA), the University of Toronto (Sunnybrook, Canada), the University of Zurich and in Solothurn (Switzerland), and at Yonsei University College (Seoul, South Korea). The University of Virginia has reported on 15 treated essential tremor patients with good clinical results [32], whereas more recently eight patients with Parkinson’s disease have been reported from Solothurn [33]. At the 3-month follow-up, the first evaluated patients enjoy an almost 60 % mean improvement on the international Unified Parkinson’s Disease Rating Scale.
Brain Tumors
Despite modern technologies, malignant brain tumors are still a serious problem for effective treatment with combined neurosurgery, chemotherapy, and radiotherapy. With the intent to treat brain tumors minimally invasively, stereotactic procedures such as laser, radiosurgery, and radio-frequency ablation have been performed for many decades. Although HIFU was used with appreciable success over 30 years ago [14], its broad acceptance as a new stereotactic modality for brain tumor surgery was hampered by the fact that a craniectomy was necessary to avoid beam scattering and skull bone heating. Using the open skull approach, Ram et al. [34] successfully treated three patients with histologically confirmed recurrent glioblastoma multiforme. MRIgFUS produced multiple histologically proven well-defined heat-induced coagulation necroses of about 50 % to almost 100 % of the respective tumor volumes.
After many years of technical development [28, 35, 36], the FDA approved the first phase I feasibility study worldwide for transcranial application of MRIgFUS. This study was subsequently initiated at Brigham and Women’s Hospital in Boston in February 2005, and the first results appeared 2010 [23••]. An ongoing tumor study at the University of Zurich, however, reveals that the treatment envelope of the 650-kHz ExAblate 4000 tcMRIgFUS system and the focal size of only a few millimeters are too small for this indication (our own experience). More recent approaches using lower ultrasound frequencies, e.g., 220 kHz, to increase the treatable volume of the brain combined with systemically applied preformed microbubbles might overcome this shortcoming [37].
Future Applications
BBB Opening and Targeted Drug Delivery
The BBB represents a complex mesh composed of endothelial cells, pericytes, and astroglial cells forming a barrier that seals the capillaries of the central nervous system (CNS) to prevent the brain from being poisoned by foreign substances circulating in the bloodstream. However, it also prevents over 98 % of neuropharmaceutical drugs from entering the CNS. A number of strategies have been developed to overcome this obstacle and to get neuropharmaceutical and chemotherapeutic agents with often large molecular size effectively to the target area inside the brain [38]. None of these methods are really satisfying. They have the disadvantage of being invasive, being effective only briefly, having toxic side effects, or opening the BBB unselectively, allowing diffuse cytotoxic drug penetration to nontargeted brain tissue [39].
Noninvasive, FUS-induced localized BBB opening offers a potential solution to the problem of transporting drugs or complex drug-carrier or gene-carrier constructs across the BBB for a multitude of CNS disorders [40]. Although application of HIFU produces focal temperature elevations to coagulate diseased brain tissue, recent preclinical studies have demonstrated that LIFU pulses combined with systemically administered preformed microbubbles, i.e., commercially available ultrasound contrast agents, are able to temporarily open the BBB in well-defined areas of the brain, thus offering enhanced drug delivery to a desired target [41••]. With use of this technique, it was possible to reversibly disrupt the BBB noninvasively under MRI guidance without associated significant tissue damage, and its integrity was restored again within hours [42, 43]. By incorporating chemotherapeutic and neuropharmacologic agents, antibodies, genes, and small interfering RNA encapsulated into nanoparticles and liposomes, this approach allows targeted drug delivery to the desired area inside the brain in a safe, reliable, and controlled manner [44••], and opens the door to a new and fascinating area for treating brain tumors and neurodegenerative and neurometabolic diseases [45, 46, 47••, 48•].
Improved Therapy for Malignant Brain Tumors and Brain Metastases
The first-line therapy for malignant brain tumors is neurosurgical resection followed by radiotherapy and chemotherapy. However, most patients eventually die of recurrent tumor. Therefore, the development of a new strategy for treatment of this dreadful disease is mandatorily required and cumulative effort aimed at developing targeted therapies for malignant glioma has been endeavored. Although radiotherapy cannot arbitrarily often be repeated, chemotherapeutic efficacy is hampered because of hindered access to the tumor by restricted penetration through the BBB, although blood vessels of most malignant brain tumors, including metastases, do not have an intact BBB. However, vasculature permeability of malignant brain tumors is heterogeneous, and infiltrating cancer cells and small metastatic seeds may be protected by vessels with intact BBB of the surrounding normal tissue, i.e., the penumbra of the tumor core [49]. For instance, in brain metastases of breast cancer, animal experiments suggest that the blood–tumor barrier is only partially compromised and that toxic concentrations of chemotherapeutic agents are only achieved in a small subset of metastases that are highly permeable [50].
Preclinical studies have demonstrated that therapeutic concentrations of chemotherapeutic agents, e.g., doxorubicin, can be achieved in the brain after noninvasive opening of the BBB locally using pulsed LIFU in the presence of preformed microbubbles [51–54]. As a consequence, a future scenario in brain tumor therapy with tcMRIgFUS might consist of a combination of bulk tumor ablation using HIFU combined with BBB opening in the penumbra of the tumor with LIFU for improved chemotherapy (Fig. 5).
Combined tcMRIgFUS brain tumor therapy using high-intensity ultrasound (HIFU) for bulk tumor ablation and low-intensity ultrasound (LIFU) for opening of the blood–brain barrier (BBB) and targeted drug therapy. (Adapted from [23••] with permission)
Since tcMRIgFUS allows the tissue temperature and exposure time to be manipulated precisely, it is well suited for applying hyperthermia to enhance the sensitivity of tumor cells as an approved adjuvant for radiotherapy or chemotherapy [55] or for targeted drug delivery with temperature-sensitive liposomes [56].
FUS-Induced Immunomodulation
Early clinical reports by Heimburger [14] and later by Ram et al. [34] describing prolonged survival of patients with malignant glioma after FUS intervention might be tentative evidence for an FUS-induced specific antitumor immune response (for a review, see [57]). A working hypothesis now accepted by the experts in the field to explain these observations postulates that the FUS-mediated tumor cell necrosis induces biological effects such as inflammatory processes, which might trigger a specific antitumor immune response. Such an FUS-induced specific antitumor immune response has been demonstrated in murine models by measuring enhanced activity of cytotoxic T lymphocytes and increased secretion of tumor-specific interferon gamma and tumor necrosis factor alpha [58, 59]. Support for this hypothesis comes from the detection of marked increases in CD3 and CD4 levels and in the T cell helper/suppressor (CD4/CD8) ratio in peripheral blood of cancer patients after HIFU treatment [59–61]. More specifically, cell debris is an endogenous danger signal and alarms the immune system against self-damage, triggering a specific antitumor response.
FUS-Induced Neuromodulation
In contrast to HIFU, the effects of short LIFU bursts on neurons and neuronal circuits are reversible [62]. After earlier in vitro experiments, recent preclinical studies using short LIFU pulses demonstrated the feasibility of controllable, bimodal—stimulatory and inhibitory—neuromodulation with FUS in vivo, e.g., suppression of rabbit visual cortex activity, or an excitatory motor response to focused ultrasound delivered to the motor cortex [63, 64••, 65]. It could even be shown that LIFU pulses can modulate the level of the neurotransmitters serotonin and dopamine [66]. LIFU pulses are delivered with no measured changes in tissue temperature at the target site or elsewhere in the brain, and no abnormal postmortem histological findings are observed. Therefore, combined with functional imaging, MRI-guided LIFU has the potential of becoming a steerable neurostimulation device for both reversible excitation and suppression of neuronal activity [67••].
Noninvasive, transcranial MRI-guided precisely targeted neuromodulation with LIFU is certainly very attractive in order to exactly explore functional targets before they are deactivated either definitely by heat coagulation methods or reversibly by electrode-mediated modulation as in deep brain stimulation. In this sense, LIFU neuromodulation could be of great value in finding optimal targets for neurofunctional disorders, such as movement disorders—essential tremor and Parkinson’s disease—as well as for epilepsy syndromes. For decades, functional neurosurgery for epilepsy has been performed using different, often rather invasive approaches with variable success. Again, FUS-mediated neuromodulation could be used to target an epileptic focus or find the optimal thalamic target before definitely treating this disabling condition. In a murine epilepsy model, LIFU has been applied to the thalamus, and its ability to reduce or even abolish epileptic activity was demonstrated [68•].
Sonothrombolysis and Stroke Therapy
There is a continuous surge for new therapeutic modalities for treating ischemic stroke and intracranial hemorrhage. In preclinical studies using the middle cerebral artery or the carotid artery as the target vessels, the use of LIFU in the presence of tissue plasminogen activator has demonstrated its potential to resolve blood clots in vivo and to recanalyze the vessel. In the CLOTBUST randomized multicenter trial of 126 patients with occlusion of the middle cerebral artery there was improved recanalization and consequently better clinical outcome without major hemorrhagic complications in patients having received sonothrombolysis [69]. However, time constrains are still a major issue when using systemic thrombolytic agents combined with mechanical clot lysis.
Conclusion
After a long history of focused ultrasound interventions to the brain for more than half a century, there are actually only a few sites that perform clinical trials on selected patients. Sophisticated technical developments for advanced MRI guidance, real-time temperature measurements, and most of all, precise transcranial ultrasound energy transmission without causing scalp burns were necessary to finally bring this novel therapeutic device as a noninvasive neurosurgical alternative to clinical acceptance. This article demonstrates the great potential of tcMRIgFUS for a variety of neurological diseases. Although many problems are still to be solved, it is hoped that transnational cooperative research efforts by neurologists, neuroradiologists, neurosurgeons, neurobiologists, physicists, and engineers will finally bring this endeavor to the best outcome for patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Heimburger RF. An encounter with stereotactic brain surgery. Neurosurgery. 2005;56(6):1367–73; discussion 1373–4.
Gillingham J. Forty-five years of stereotactic surgery for Parkinson’s disease: a review. Stereotact Funct Neurosurg. 2000;74(3–4):95–8.
Pereira EA, Green AL, Nandi D, Aziz TZ. Stereotactic neurosurgery in the United Kingdom: the hundred years from Horsley to Hariz. Neurosurgery. 2008;63(3):594–606; discussion 606–7.
Rahman M, Murad GJ, Mocco J. Early history of the stereotactic apparatus in neurosurgery. Neurosurg Focus. 2009;27(3):E12.
Warwick R, Pond J. Trackless lesions in nervous tissues produced by high intensity focused ultrasound (high-frequency mechanical waves). J Anat. 1968;102(Pt 3):387–405.
Jolesz FA, Hynynen KH. MRI-guided focused ultrasound surgery. New York: Informa Healthcare; 2007.
Lynn JG, Zwemer RL, Chick AJ. The biological application of focused ultrasonic waves. Science. 1942;96(2483):119–20.
Lynn JG, Putnam TJ. Histology of cerebral lesions produced by focused ultrasound. Am J Pathol. 1944;20(3):637–49.
Fry FJ, Ades HW, Fry WJ. Production of reversible changes in the central nervous system by ultrasound. Science. 1958;127(3289):83–4.
Fry WJ. Intense ultrasound: a new tool for neurological research. J Ment Sci. 1954;100(418):85–96.
Fry WJ, Barnard JW, Fry EJ, Krumins RF, Brennan JF. Ultrasonic lesions in the mammalian central nervous system. Science. 1955;122(3168):517–8.
Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron. 1960;ME-7:166–81.
Lele PP. A simple method for production of trackless focal lesions with focused ultrasound: physical factors. J Physiol. 1962;160:494–512.
Heimburger RF. Ultrasound augmentation of central nervous system tumor therapy. Indiana Med. 1985;78(6):469–76.
Meyers R, Fry FJ, Fry WJ, Eggleton RC, Schultz DF. Determination of topological human brain representations and modifications of signs and symptoms if some neurologic disorders by the use of high level ultrasound. Neurology. 1960;10(3):271–7.
Meyers R, Fry WJ, Fry FJ, Dreyer LL, Schultz DF, Noyes RF. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J Neurosurg. 1959;16(1):32–54.
Lindstrom PA. Prefrontal ultrasonic irradiation—a substitute for lobotomy. AMA Arch Neurol Psychiatry. 1954;72(4):399–425.
Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol. 1998;24(2):275–83.
Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol. 2002;47(8):1219–36.
Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am. 2003;113(1):84–93.
•• Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med. 2009;60:417–30. This is a comprehensive overview of clinical applications of MRI-guided focused ultrasound.
•• Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66(6):858–61. This is the first clinical study on focused ultrasound brain interventions in functional brain disorders.
•• McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66(2):323–32; discussion 332. This is the first clinical study on brain tumor surgery with MRI-guided focused ultrasound.
• Jeanmonod D, Werner B, Morel A, et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus. 2012;32(1):E1. This is an extension of our clinical study on FUS for treatment of neurofunctional disorders.
Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–94.
Miller DL. A review of the ultrasonic bioeffects of microsonation, gas-body activation, and related cavitation-like phenomena. Ultrasound Med Biol. 1987;13(8):443–70.
McDannold N, Moss M, Killiany R, et al. MRI-guided focused ultrasound surgery in the brain: tests in a primate model. Magn Reson Med. 2003;49(6):1188–91.
Hynynen K, Clement GT, McDannold N, et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: a preliminary rabbit study with ex vivo human skulls. Magn Reson Med. 2004;52(1):100–7.
Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34(6):814–23.
Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging. 2008;27(2):376–90.
Morel A. Stereotactic atlas of the human thalamus and basal ganglia. New York: Informa Healthcare; 2007.
Elias J, Huss D, Khaled M, et al. MR guided focused ultrasound lesioning for the treatment of essential tremor. A new paradigm for noninvasive lesioning and neuromodulation. In: Congress of neurological surgeons annual meeting, Washington; 2011.
Jeanmonod D, Moser D, Magara A, et al. Study of incisionless transcranial magnetic resonance-guided focused ultrasound treatment of Parkinson’s disease: safety, accuracy and initial outcomes. In: 3rd international symposium on focused ultrasound. Washington; 2012.
Ram Z, Cohen ZR, Harnof S, et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery. 2006;59(5):949–55; discussion 955–6.
Hynynen K, Vykhodtseva NI, Chung AH, Sorrentino V, Colucci V, Jolesz FA. Thermal effects of focused ultrasound on the brain: determination with MR imaging. Radiology. 1997;204(1):247–53.
Clement GT, White J, Hynynen K. Investigation of a large-area phased array for focused ultrasound surgery through the skull. Phys Med Biol. 2000;45(4):1071–83.
McDannold N, Livingstone M, Arvanitis C, Zhang Y, Jolesz F, Vykhodtseva N. Non-thermal ablation in the brain via focused ultrasound combined with an ultrasound contrast agent: long-term treatment effects and feasibility in a large animal model. In: 3rd international symposium on focused ultrasound. Washington; 2012.
Pardridge WM. Biopharmaceutical drug targeting to the brain. J Drug Target. 2010;18(3):157–67.
Kemper EM, Boogerd W, Thuis I, Beijnen JH, van Tellingen O. Modulation of the blood-brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours? Cancer Treat Rev. 2004;30(5):415–23.
Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics. 2008;48(4):279–96.
•• Choi JJ, Selert K, Gao Z, Samiotaki G, Baseri B, Konofagou EE. Noninvasive and localized blood-brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies. J Cereb Blood Flow Metab. 2010;31(2):725–37. This is experimental study on BBB opening with FUS.
Hynynen K, McDannold N, Vykhodtseva N, et al. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105(3):445–54.
Baseri B, Choi JJ, Tung YS, Konofagou EE. Multi-modality safety assessment of blood–brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultrasound Med Biol. 2010;36(9):1445–59.
•• McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72(14):3652–63. This is an important preclinical study on BBB opening using FUS in combination with microbubbles.
Etame AB, Diaz RJ, Smith CA, Mainprize TG, Hynynen K, Rutka JT. Focused ultrasound disruption of the blood–brain barrier: a new frontier for therapeutic delivery in molecular neurooncology. Neurosurg Focus. 2012;32(1):E3.
Huang Q, Deng J, Wang F, et al. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp Neurol. 2012;233(1):350–6.
•• Burgess A, Ayala-Grosso CA, Ganguly M, Jordao JF, Aubert I, Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS ONE. 2011;6(11):e27877. This is a pioneering preclinical study on targeted delivery of stem cells to the brain.
• Burgess A, Huang Y, Querbes W, Sah DW, Hynynen K. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J Control Release. 2012;163(2):125–9. This provides a demonstration of effective gene therapy with targeted delivery of small interfering RNA after BBB opening.
Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8(6):344–56.
Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood–tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–78.
Park J, Zhang Y, Vykhodtseva N, Jolesz FA, McDannold NJ. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J Control Release. 2012;162(1):134–42.
Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121(4):901–7.
Yang FY, Teng MC, Lu M, et al. Treating glioblastoma multiforme with selective high-dose liposomal doxorubicin chemotherapy induced by repeated focused ultrasound. Int J Nanomed. 2012;7:965–74.
Kovacs Z, Martin E, Bernasconi M, Werner B. Focused ultrasound-mediated delivery of doxorubicin in a mouse model of glioblastoma. In: Symposium of the International Society for Therapeutic Ultrasound. Heidelberg; 2012.
Guthkelch AN, Carter LP, Cassady JR, et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial. J Neurooncol. 1991;10(3):271–84.
Gasselhuber A, Dreher MR, Partanen A, et al. Targeted drug delivery by high intensity focused ultrasound mediated hyperthermia combined with temperature-sensitive liposomes: computational modelling and preliminary in vivo validation. Int J Hyperthermia. 2012;28(4):337–48.
Haen SP, Pereira PL, Salih HR, Rammensee HG, Gouttefangeas C. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol. 2011;2011:160250.
Hu Z, Yang XY, Liu Y, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med. 2007;5:34.
Xia JZ, Xie FL, Ran LF, Xie XP, Fan YM, Wu F. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med Biol. 2012;38(8):1363–71.
Wu F, Wang ZB, Lu P, et al. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol. 2004;30(9):1217–22.
Deng J, Zhang Y, Feng J, Wu F. Dendritic cells loaded with ultrasound-ablated tumour induce in vivo specific antitumour immune responses. Ultrasound Med Biol. 2010;36(3):441–8.
Tufail Y, Yoshihiro A, Pati S, Li MM, Tyler WJ. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat Protoc. 2011;6(9):1453–70.
Tyler WJ. Noninvasive neuromodulation with ultrasound? A continuum mechanics hypothesis. Neuroscientist. 2011;17(1):25–36.
•• Yoo SS, Bystritsky A, Lee JH, et al. Focused ultrasound modulates region-specific brain activity. Neuroimage. 2011;56(3):1267–75. This reports that reversible neuromodulation with FUS is an important noninvasive tool for neurology.
Yoo SS, Kim H, Min BK, Franck E, Park S. Transcranial focused ultrasound to the thalamus alters anesthesia time in rats. Neuroreport. 2011;22(15):783–7.
Min BK, Yang PS, Bohlke M, et al. Focused ultrasound modulates the level of cortical neurotransmitters: potential as a new functional brain mapping technique. Int J Imaging Syst Technol. 2011;21:232–40.
•• Bystritsky A, Korb AS, Douglas PK, et al. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011;4(3):125–36. This is important review article to understand the mechanisms of FUS-induced neuromodulation.
• Min BK, Bystritsky A, Jung KI, et al. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci. 2011;12:23. This is pioneering preclinical work on neuromodulation of epilepsy.
Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351(21):2170–8.
Disclosure
No potential conflict of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, E., Werner, B. Focused Ultrasound Surgery of the Brain. Curr Radiol Rep 1, 126–135 (2013). https://doi.org/10.1007/s40134-013-0013-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40134-013-0013-0