Abstract
Introduction
To evaluate the real-world efficacy of aflibercept using the treat-and-extend (TnE) regimen in treating neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV), and to analyze biomarkers using optical coherence tomography (OCT) to predict treatment outcomes.
Methods
Patients diagnosed with nAMD or PCV who received an intravitreal injection of aflibercept following the TnE regimen for ≥ 2 years were retrospectively reviewed. Data on best-corrected visual acuity (BCVA), number of injections, treatment interval, and OCT biomarkers, including central macular thickness, presence of subretinal fluid (SRF), and serous pigmented epithelial detachment, were collected at baseline and at 3, 6, 12, 18, and 24 months after the first injection.
Results
A total of 43 patients were enrolled in this study, 24 of whom were diagnosed with nAMD and 19 with PCV. The BCVA in logMAR (mean ± standard deviation) improved from 0.75 ± 0.41 (baseline) to 0.60 ± 0.41 (P = 0.002) at 3 months after treatment initiation, and further improved to 0.66 ± 0.46 at 24 months (P = 0.137). The number of injections (mean ± standard deviation) within the 2-year treatment course was 10.95 ± 3.65. At month 24 of the TnE regimen, the treatment interval was extended to ≥ 16 weeks in 60.5% of all cases and to 78.9% of the PCV cases. After three loading injections, persistent subretinal fluid and intraretinal fluid were predictive of more frequent injections (P = 0.026) and poorer visual outcomes (P = 0.050), respectively.
Conclusion
Aflibercept combined with a TnE regimen was effective in treating nAMD and PCV in a real-world setting. The treatment interval could be extended to ≥ 16 weeks in 60.5% of the cases after a 2-year treatment regimen. OCT can be used to predict the treatment course and visual outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Aflibercept with a treat-and-extend (TnE) regimen and a treatment interval of up to 16 weeks has been proven to be effective in the treatment of age-related macular degeneration in clinical trials, but the results in a real-world setting with a treatment interval > 16 weeks have rarely been reported. |
The effect of aflibercept on neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV) have seldom been compared in previous reports. |
What was learned from the study? |
Aflibercept with a TnE regimen for nAMD and PCV in real-world practice could achieve favorable outcomes comparable to those reported in clinical trials. |
Cases with PCV could maintain the treatment effect with fewer injections and longer treatment intervals than those with nAMD after the 2-year treatment courses, although the case numbers were relatively small in this study. |
The presence of subretinal fluid after the loading treatment indicated a more refractory course that necessitated more injections; however, similar visual outcomes could be achieved at 2 years for cases with or without subretinal fluid after the loading treatment. |
Introduction
Age-related macular degeneration (AMD) is the leading cause of vision-threatening diseases in developed countries, and neovascular AMD (nAMD) and geographic atrophy are the two main causes of vision loss in AMD [1]. Polypoidal choroidal vasculopathy (PCV) has been recognized as a subtype of AMD if polyps are present in the subretinal neovascular network [2]; however, nowadays, PCV is considered a spectrum of pachychoroid diseases [3]. PCV is more common in Asian populations than in Western populations, while risk factors, including older age and the presence of diabetes, are more associated with AMD than with PCV [3]. Moreover, patients with PCV show more favorable outcomes than those with nAMD because of the lower chance of macular scar formation during the natural course [3].
While photodynamic therapy and laser photocoagulation are less effective in preventing visual deterioration following the natural history of nAMD and PCV, the introduction of anti-vascular endothelial growth factor (VEGF) has given ophthalmologists hope to slow disease progression [4]. However, the treatment protocols in early clinical trials, which required monthly follow-up and persistent injection at least every 2 months after the loading treatment [4, 5], are difficult to implement in a real-world clinical setting. The potential for extending the treatment interval of anti-VEGF injections has been proven in both reactive pro re nata (PRN) and proactive treat-and-extend (TnE) protocols after three monthly loading injections with good treatment efficacy [6, 7]. For aflibercept, the clinical trial ALTAIR revealed that visual improvement was maintained in more than half of the cases with a treatment interval of at least 12 weeks, and in more than 40% with a 16-week treatment interval, at 2 years when following the TnE protocol for nAMD, including PCV [8]. However, there are no real-world reports of patients with nAMD or PCV receiving aflibercept treatment using the TnE protocol for up to a 16-week treatment interval.
To optimize the treatment efficacy, several biomarkers on optical coherence tomography (OCT) have been used to predict the visual outcomes of nAMD [9]. Presence of intraretinal fluid (IRF) is considered a negative prognostic factor in most clinical studies, whereas the presence of subretinal fluid (SRF) has sometimes been correlated with a relatively benign disease course [10]. However, the relationship between visual prognosis and the location or amount of SRF has not yet been elucidated. Different studies have been designed to tolerate different amounts of SRF when vision is maintained [8, 11, 12]. Moreover, the presence of pigmented epithelial detachment (PED), especially serous PED (SPED), which represents disease activity, has not been yet discussed thoroughly[10, 13, 14]. For real-world practice, the predictive ability of OCT biomarkers is even more controversial because of diverse clinical situations.
In the present study, we aimed to investigate the 2-year real-world treatment results for patients with nAMD or PCV treated with aflibercept using a TnE protocol of up to more than a 16-week treatment interval in a tertiary center, and to identify possible biomarkers using OCT.
Methods
Study Population
The medical records of patients who had been diagnosed with nAMD or PCV and treated with 2 mg aflibercept (Eylea®; Bayer, Basel, Switzerland) for at least 2 years at the National Taiwan University Hospital between January 2016 and March 2022 were retrospectively reviewed. Patients treated with three monthly loading injections of aflibercept, followed by a TnE protocol, were included in this study. The exclusion criteria included a history of glaucoma, geographic atrophy at baseline, coexisting retinal vascular disease or vitreomaculopathy, and previous anti-VEGF treatment for AMD or PCV within 6 months. If both eyes of the same patient met the inclusion criteria, only the more severely affected eye was included. The criteria for the TnE protocol were based on the consensus of Taiwan Retina Society, except that the maximum treatment interval could be ≥ 16 weeks according to physicians’ decision [15]. Briefly, the treatment criteria are as follows. If the best-corrected visual acuity (BCVA) remained stable with < 1 line loss and the retina was dry (no IRF or SRF) on OCT after the loading phase, the treatment interval was extended by 2–4 weeks. If the retina was not completely dry but the fluid was stationary or decreased, the treatment interval was maintained. The treatment interval was shortened if there was an increase in fluid, new hemorrhage, or loss of BCVA for ≥ 1 line. The minimal treatment interval was 4 weeks, and the TnE regimen was maintained without an exit strategy during the 2-year treatment period.
This study was approved by the Institutional Review Board of the National Taiwan University Hospital (No. 202212148RINA), and adhered to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study.
Data Collection
We collected baseline clinical data, including age, sex, previous anti-VEGF treatment history, BCVA in logarithm of the minimum angle of resolution (LogMAR), fundus photography, fluorescein angiography (FA), indocyanine green angiography (ICGA), and OCT images. OCT was performed using the RTVue XR Avanti with the AngioVue OCTA system (Optovue Inc., Fremont, CA, USA), and FA and ICGA were performed using Heidelberg retinal angiography HRA 2 system (Heidelberg Engineering Inc., Heidelberg, Germany). Diagnoses of nAMD and PCV were established according to FA and ICGA images by two retinal specialists (Y-TH and C-HH). The central macular thickness (CMT, the mean retinal thickness of the 1 mm-diameter area of central fovea) and the presence of IRF, SRF, and SPED (serous pigmented epithelium detachment) within 3 mm of the central fovea on OCT were recorded. The following data were collected at 3, 6, 12, 18, and 24 months after the first injection: BCVA in logMAR; CMT; number of injections; and the presence of IRF, SRF, and SPED on OCT.
Statistical Analysis
Numeric values are presented as mean ± standard deviation. For the analysis of BCVA in logMAR and CMT, comparisons of measurements between the baseline and follow-up visits were performed using Wilcoxon signed-rank tests. Chi-square (χ2) and Wilcoxon rank-sum test were performed to compare the parameters between the nAMD and PCV groups. Linear regression analyses were performed to evaluate the correlating factors for logMAR BCVA at 12 and 24 months after the initial injection and the number of injections at 24 months. Logistic regression analysis was performed to evaluate the correlating factors for treatment intervals ≥ 16 weeks at 24 months. All the statistical analyses were performed using SPSS version 21 (IBM Corp., Armonk, NY, USA). Statistical significance was set at P < 0.05.
Results
Baseline Characteristics
A total of 43 patients were enrolled in this study, 24 of whom were diagnosed with AMD and 19 with PCV. The mean age was 69.4 ± 9.7 years, and 24 of the patients (55.8%) were men. Patients with PCV were younger than those with nAMD (P = 0.033). The mean logMAR of BCVA at baseline was 0.75 ± 0.41, and the mean baseline CMT was 273.02 ± 86.05 μm. The proportions of IRF, SRF, and SPED at baseline were 7.0%, 65.1%, and 41.9%, respectively. No significant differences in baseline characteristics were observed between the nAMD and PCV groups (P > 0.05), except that the proportion of SRF was higher in the PCV group (84.2%) than in the nAMD group (50.0%) (P = 0.026). The demographic data and baseline characteristics are listed in Table 1.
Visual and Anatomical Outcomes
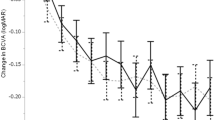
After three loading injections, the overall mean LogMAR of BCVA significantly improved from 0.75 ± 0.41 at baseline to 0.60 ± 0.41 at 3 months (P = 0.002). The PCV and nAMD groups showed similar visual improvements after three loading injections. Vision improvement could be maintained up to 12 months, when the overall mean LogMAR of BCVA was 0.61 ± 0.45 (P = 0.038), but the BCVA then declined gradually to 0.66 ± 0.46 at 24 months, resulting in no further significant visual improvement (P = 0.14). For the PCV patient group, vision kept improving after 3 months and remained rather stable; however, for the wAMD group, vision declined after 3 months, although no significant difference in visual improvement between two groups was noted at 12 months (− 0.20 ± 0.60 for the PCV group and − 0.09 ± 0.36 for the nAMD group; P = 0.62) and 24 months (− 0.15 ± 0.64 for the PCV group and - 0.04 ± 0.34 for the nAMD group; P = 0.54) (Fig. 1a). Regarding the anatomical outcome, the mean CMT significantly decreased from 273.02 ± 86.05 μm at baseline to 225.11 ± 55.41 μm at 3 months after the loading treatment (P < 0.001), and remained stable at 12 months (226.95 ± 61.93 μm) and 24 months (224.14 ± 56.45 μm), with significant decrease compared to baseline (P < 0.001 for both) (Fig. 1b). Similarly, the decrease in CMT at 24 months was more obvious in the PCV group than in the nAMD group (− 75.37 ± 90.82 μm and − 27.92 ± 52.65 μm, respectively; P = 0.091).
a Changes in visual acuity in LogMAR of best-corrected visual acuity (BCVA) from baseline to 24 months after aflibercept treatment with a treat-and-extend regimen for all enrolled cases, and further subgrouping as neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV). Asterisks indicate significance at *P = 0.002, **P = 0.019, and ***P = 0.038; the hash sign indicates significance at #P = 0.005. b Changes in central macular thickness (CMT) from baseline to 24 months after treatment. Compared to the baseline status, the CMTs decreased significantly at all time points for all groups (P < 0.05 for all)
Injection Number and Treatment Interval at 12 and 24 Months
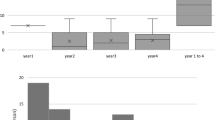
The average number of injections was 7.07 ± 1.28 and 10.95 ± 3.65 within 12 and 24 months, respectively. Patients in the PCV group received fewer injections than those in the nAMD group at 24 months (P = 0.006). Twelve months after the first injection, 41.8% and 11.6% of the patients achieved injection intervals ≥ 12 weeks and ≥ 16 weeks, respectively. At 24 months after the first injection, 69.8% and 60.5% of the patients had achieved injection intervals of ≥ 12 weeks and ≥ 16 weeks, respectively. The maximum treatment interval was 24 weeks at 24 months. For patients with PCV, the proportions of patients achieving a treatment interval ≥ 12 weeks (84.2%) and ≥ 16 weeks (78.9%) at 24 months were even higher, although no significant differences between the nAMD and PCV groups were noted (P = 0.21) (Table 2).
Correlating Factors for Visual Outcomes
Both older age and poor vision at baseline were predictive factors for poorer vision at 12 months (P = 0.008 and P = 0.009, respectively) and 24 months (P = 0.004 and P = 0.014, respectively), although poor VA at baseline was also correlated with better visual improvement at 12 and 24 months (P = 0.001 for both time points). Regarding the OCT biomarkers, the presence of IRF at baseline correlated with poorer BCVA at 12 months (P = 0.050), and the presence of persistent IRF at 3 months correlated with poorer BCVA at 24 months (P = 0.050). The presence of SRF at 24 months was correlated with poorer BCVA (P = 0.004). The presence of SPED at any time point did not correlate with the visual outcomes (Table 3).
Correlating Factors for Treatment Interval and Injection Number at 24 Months
Younger patients (P = 0.027) with absence of fluid, including those with IRF (P = 0.013), SRF (P = 0.024), and SPED (P = 0.011) at 24 months tended to have longer treatment interval at 24 months. Younger patients (P = 0.003) with absence of SRF (P = 0.026) or SPED (P = 0.026) also had fewer injections at 24 months. Those with persistent SRF at 3 months tended to have more injections and a shorter treatment interval at 24 months (P = 0.026 and P = 0.005, respectively) (Table 4).
Discussion
With the advancement of anti-VEGF treatment in AMD and PCV, the TnE regimen has become widely used in daily practice and is considered beneficial for balancing treatment efficacy and loading [16, 17]. Several prospective studies have achieved the optimal treatment outcome by using the TnE regimen (either ranibizumab or aflibercept) [8, 11, 18]. However, there is a lack of evidence for direct head-to-head comparison between these two drugs with the TnE regimen. Although the results of these studies cannot be directly compared with one another, it appears that a longer treatment interval can be achieved with aflibercept. A network meta-analysis revealed similar visual outcomes for both drugs but with fewer aflibercept injections [19]. The RIVAL study revealed similar outcomes for ranibizumab and aflibercept, but the number of injections was higher than that reported in other studies owing to the stricter study design [20]. When the concentration of VEGF in the anterior chamber was tested, the suppressive effect was more stable with aflibercept [21]. This finding possibly explains why aflibercept could achieve a longer treatment effect than ranibizumab using the TnE regimen in the meta-analysis.
One of the advantages of the TnE regimen for anti-VEGF therapy is that the treatment outcomes in clinical trials can be replicated in real-world settings. Lukic et al. [22] reported the results of 4 years of clinical experience in the UK, showing that a one-line improvement in Snellen VA could be maintained through 4 years with 19.3 injections. In this study cohort, approximately one-third of the cases did not need to receive injections over years 3 and 4, and most of these cases were absent from disease recurrence after reaching an injection-free status [22]. Maruko et al. reported the results of a 2-year prospective study of the TnE regimen in Japan, in which half of the cases were diagnosed with PCV. Visual improvement of 0.13 in logMAR was achieved in 2 years with 13.8 injections, and 60.8% of the cases had an injection interval of > 12 weeks [23]. In a Swiss study presented by Jaggi et al., visual improvement was maintained for 4 years, and 35% of the cases were injection-free at the end of the follow-up [24]. In the present study, the treatment interval could be elongated to ≥ 16 weeks in 60.5% of the cases, with the mean visual improvement of 0.09 in logMAR and an average of 10.95 injections in 2 years. To evaluate the efficacy of the TnE regimen, we enrolled only cases strictly following the TnE regimen without an exit strategy during the first 2 years of treatment in our study. The visual improvement was comparable with that of the UK real-world study and relatively inferior to that of prospective TnE studies, such as the ALTAIR and Japanese studies. In contrast, more patients in our study achieved a 16-week treatment interval with fewer injections, which reflects the relatively loosened treatment criteria in a real-world setting.
The key elements for determining the treatment interval in the TnE protocol are visual acuity and fluid status. By evaluating fluid-related biomarkers on OCT, which represent disease activity, we can extend, maintain or shorten the treatment interval accordingly. Therefore, the association between OCT biomarkers and visual acuity should be elucidated to validate this protocol. In previous prospective studies, the presence and amount of IRF were consistently considered to be poor prognostic factors for visual outcomes, and the presence of PED was associated with disease reactivation, a higher number of injections, and long-term visual loss [9, 10, 25]. While many previous prospective studies identified the presence of SRF as a good prognostic factor for visual outcome, several studies also reported different results when taking SRF volume into consideration. Ying et al. [26] found that cases with SRF < 25 μm had better visual improvement in their post hoc analysis of the CATT study. Busbee et al. [27] found that cases with SRF > 118 μm required more injections in the post-hoc analysis of the HARBOR study. Moreover, recurrent SRF during treatment has a negative impact on visual outcomes [9]. In our study, we found that the presence of SRF at 24 months was correlated with poorer BCVA. Moreover, the presence of SRF after the loading injections at 3 months was correlated with a higher number of injections and shorter treatment interval, but not with poorer BCVA at 24 months. This results demonstrates that persistent SRF after the loading phase indicates a more refractory course that necessitates more frequent injections; however, vision could be maintained if the SRF resolved after aggressive treatment with the TnE regimen. In contrast, Siedlecki et al. found that the presence of SRF was associated with a lower rate of progression to geographic atrophy and prevented long-term visual loss [28]. However, geographic atrophy is relatively uncommon in Asian populations compared to Caucasians [29]. This may explain why we did not observe a protective effect of SRF in the present study. Consensus has been reached by many expert committees for refining the TnE regimen by incorporating the above-mentioned results of fluid biomarker analysis [9, 15, 30].
PCV is commonly regarded as one of the pachychoroid spectrum diseases as well as a special subtype of nAMD and is more prevalent in Asian populations, with a reported prevalence ranging from 22.3% to 61.6%, than in Caucasians [3]. Compared to the risk factors between typical nAMD and PCV, Sakurada et al. found younger age and fewer comorbidities with diabetes and end-stage renal disease in PCV cases [31]. The difference between nAMD and PCV also exists in the response to anti-VEGF treatment. Maruko et al. reported a prospective study on the treatment of nAMD and PCV in Japanese populations and found that patients with PCV had greater CMT reduction, fewer injections, and a higher proportion of longer injection intervals [23]. These findings were in accordance with those of the present study, in which 78.9% of PCV cases achieved a 16-week treatment interval at the end of the second year, with an average improvement of 0.15 logMAR in BCVA. These results suggest that aflibercept monotherapy with the TnE regimen is suitable for treating PCV.
The major limitations of our study are its retrospective nature and possible selection bias due to non-adherence or non-compliance with the TnE regimen. Furthermore, the number of cases enrolled in the study was relatively small due to the strict TnE criteria. These factors resulted in little significant differences being observed between the nAMD and PCV groups. However, this is one of the few published real-world case series of patients with PCV treated with aflibercept in the TnE regimen. A longer follow-up period and quantitative evaluation of OCT biomarkers in future studies would further clarify the predictive factors for the treatment outcomes of aflibercept combined with a TnE regimen in AMD and PCV cases.
Conclusions
In conclusion, we found that a TnE regimen with aflibercept was effective in treating nAMD or PCV in real-world practice. Both anatomical and functional improvements were achieved in 2 years with an average of 10.95 injections. The treatment interval was extended to > 16 weeks in 60.5% of all the cases and in 78.9% of the PCV cases. After three loading injections, persistent SRF was predictive of more frequent injections and persistent IRF was predictive of poorer visual outcomes.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–38.
Lim LS, Cheung CM, Wong TY. Asian age-related macular degeneration: current concepts and gaps in knowledge. Asia Pac J Ophthalmol (Phila). 2013;2(1):32–41.
Wong CW, Yanagi Y, Lee WK, et al. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res. 2016;53:107–39.
Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57-65 e5.
Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48.
Group CR, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–908.
Haga A, Kawaji T, Ideta R, Inomata Y, Tanihara H. Treat-and-extend versus every-other-month regimens with aflibercept in age-related macular degeneration. Acta Ophthalmol. 2018;96(3):e393–8.
Ohji M, Takahashi K, Okada AA, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52-and 96-week findings from ALTAIR: a randomized controlled trial. Adv Ther. 2020;37(3):1173–87.
Ashraf M, Souka A, Adelman RA. Age-related macular degeneration: using morphological predictors to modify current treatment protocols. Acta Ophthalmol. 2018;96(2):120–33.
Wong DT, Berger AR, Bourgault S, et al. Imaging biomarkers and their impact on therapeutic decision-making in the management of neovascular age-related macular degeneration. Ophthalmologica. 2021;244(4):265–80.
Mitchell P, Holz FG, Hykin P, et al. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: the aries study: a randomized clinical trial. Retina. 2021;41(9):1911–20.
Guymer RH, Markey CM, McAllister IL, et al. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology. 2019;126(5):723–34.
Lai TT, Hsieh YT, Yang CM, Ho TC, Yang CH. Biomarkers of optical coherence tomography in evaluating the treatment outcomes of neovascular age-related macular degeneration: a real-world study. Sci Rep. 2019;9(1):529.
Tarakcioglu HN, Ozkaya A, Kemer B, Taskapili M. Multimodal imaging based biomarkers predictive of early and late response to anti-VEGFs during the first year of treatment for neovascular age-related macular degeneration. J Fr Ophtalmol. 2019;42(1):22–31.
Cheng CK, Chen SJ, Chen JT, et al. Optimal approaches and criteria to treat-and-extend regimen implementation for neovascular age-related macular degeneration: experts consensus in Taiwan. BMC Ophthalmol. 2022;22(1):25.
Aurell S, Sjovall K, Paul A, Moren A, Granstam E. Better visual outcome at 1 year with antivascular endothelial growth factor treatment according to treat-and-extend compared with pro re nata in eyes with neovascular age-related macular degeneration. Acta Ophthalmol. 2019;97(5):519–24.
Barthelmes D, Nguyen V, Daien V, et al. Two year outcomes of “treat and extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina. 2018;38(1):20–8.
Kertes PJ, Galic IJ, Greve M, et al. Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA Ophthalmol. 2020;138(3):244–50.
Ohji M, Lanzetta P, Korobelnik JF, et al. Efficacy and treatment burden of intravitreal aflibercept versus intravitreal ranibizumab treat-and-extend regimens at 2 years: network meta-analysis incorporating individual patient data meta-regression and matching-adjusted indirect comparison. Adv Ther. 2020;37(5):2184–98.
Gillies MC, Hunyor AP, Arnold JJ, et al. Macular atrophy in neovascular age-related macular degeneration: a randomized clinical trial comparing ranibizumab and aflibercept (RIVAL study). Ophthalmology. 2020;127(2):198–210.
Fauser S, Muether PS. Clinical correlation to differences in ranibizumab and aflibercept vascular endothelial growth factor suppression times. Br J Ophthalmol. 2016;100(11):1494–8.
Lukic M, Eleftheriadou M, Hamilton RD, Rajendram R, Bucan K, Patel PJ. Four-year outcomes of aflibercept treatment for neovascular age-related macular degeneration: results from real-life setting. Eur J Ophthalmol. 2021;31(4):1940–4.
Maruko I, Ogasawara M, Yamamoto A, et al. Two-year outcomes of treat-and-extend intravitreal aflibercept for exudative age-related macular degeneration: a prospective study. Ophthalmol Retina. 2020;4(8):767–76.
Jaggi D, Nagamany T, Ebneter A, Munk M, Wolf S, Zinkernagel M. Aflibercept for age-related macular degeneration: 4-year outcomes of a “treat-and-extend” regimen with exit-strategy. Br J Ophthalmol. 2022;106(2):246–50.
Waldstein SM, Philip AM, Leitner R, et al. Correlation of 3-dimensionally quantified intraretinal and subretinal fluid with visual acuity in neovascular age-related macular degeneration. JAMA Ophthalmol. 2016;134(2):182–90.
Ying GS, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122–9.
Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046–56.
Siedlecki J, Fischer C, Schworm B, et al. Impact of sub-retinal fluid on the long-term incidence of macular atrophy in neovascular age-related macular degeneration under treat & extend anti-vascular endothelial growth factor inhibitors. Sci Rep. 2020;10(1):8036.
Rim TH, Kawasaki R, Tham YC, et al. Prevalence and pattern of geographic atrophy in asia: the Asian eye epidemiology consortium. Ophthalmology. 2020;127(10):1371–81.
Ross AH, Downey L, Devonport H, et al. Recommendations by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration. Eye (Lond). 2020;34(10):1825–34.
Sakurada Y, Yoneyama S, Imasawa M, Iijima H. Systemic risk factors associated with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Retina. 2013;33(4):841–5.
Funding
No funding was received for conducting this study and the journal’s Rapid Service fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, and data collection and analysis were performed by Chu-Hsuan Huang, Tso-Ting Lai, and Chang-Hao Yang. The first draft of the manuscript was written by Chu-Hsuan Huang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.”
Corresponding author
Ethics declarations
Conflict of Interest
Chu-Hsuan Huang, Tso-Ting Lai, Chang-Hao Yang and Yi-Ting Hsieh received speaker honoraria from The Bayer Taiwan.
Ethical Approval
This study was approved by the Institutional Review Board of the National Taiwan University Hospital (No. 202212148RINA) and adhered to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Huang, CH., Lai, TT., Yang, CH. et al. Two-Year Real-World Results for Aflibercept Using the Treat-and-Extend Regimen in Neovascular Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy. Ophthalmol Ther 13, 385–396 (2024). https://doi.org/10.1007/s40123-023-00850-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00850-6