Abstract
Introduction
We studied the correlation of central macular fluid volume (CMFV) and central subfield thickness (CST) with best-corrected visual acuity (BCVA) in treatment-naïve eyes with diabetic macular edema (DME) 1 month after anti-vascular endothelial growth factor (VEGF) therapy.
Methods
This retrospective cohort study investigated eyes that received anti-VEGF therapy. All participants underwent comprehensive examinations and optical coherence tomography (OCT) volume scans at baseline (M0) and 1 month after the first treatment (M1). Two deep learning models were separately developed to automatically measure the CMFV and the CST. Correlations were analyzed between the CMFV and the logMAR BCVA at M0 and logMAR BCVA at M1. The area under the receiver operating characteristic curve (AUROC) of CMFV and CST for predicting eyes with BCVA \(\ge\) 20/40 at M1 was analyzed.
Results
This study included 156 DME eyes from 89 patients. The median CMFV decreased from 0.272 (0.061–0.568) at M0 to 0.096 (0.018–0.307) mm3 at M1. The CST decreased from 414 (293–575) to 322 (252–430) μm. The logMAR BCVA decreased from 0.523 (0.301–0.817) to 0.398 (0.222–0.699). Multivariate analysis demonstrated that the CMFV was the only significant factor for logMAR BCVA at both M0 (β = 0.199, p = 0.047) and M1 (β = 0.279, p = 0.004). The AUROC of CMFV for predicting eyes with BCVA \(\ge\) 20/40 at M1 was 0.72, and the AUROC of CST was 0.69.
Conclusions
Anti-VEGF therapy is an effective treatment for DME. Automated measured CMFV is a more accurate prognostic factor than CST for the initial anti-VEGF treatment outcome of DME.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Automated fluid volume measurements for eyes with diabetic macular edema (DME) using optical coherence tomography (OCT) remains a challenge. |
In this study, two deep learning models were separately developed to automatically measure central macular fluid volume (CMFV) and central subfield thickness (CST). |
The results indicated that CMFV is a more accurate prognostic factor than CST for the initial anti-VEGF treatment outcome of DME. |
This study may improve the supervision and prediction of vision in eyes with DME. |
Introduction
Diabetic macular edema (DME) is the leading cause of vision loss in patients with diabetic retinopathy and is gradually becoming a global health problem [1]. The prevalence of DME is estimated to be between 4.2% and 7.9% among patients with type 1 diabetes and 1.4% to 12.8% among type 2 diabetes [2]. The onset of DME is associated with a number of factors including duration of diabetes, degree of metabolic control, elevated glycosylated hemoglobin A1C (HbA1c), severity of retinopathy, hypertension, socioeconomic status, and age [3]. Anti-vascular endothelial growth factor (anti-VEGF) therapy is extremely effective at preserving visual function and has become the leading treatment approach for DME [4]. However, there are still large individual differences in the efficacy of anti-VEGF treatment [5]. At the same time, frequent intravitreal injections increase the risk of complications, the financial burden on patients, and the waste of social resources [6]. Therefore, the search for an accurate prognostic factor of anti-VEGF treatment in DME patients to optimize the treatment strategy is a research hotspot.
Optical coherence tomography (OCT) makes it possible to deliver a considerable amount of morphological information through high-resolution raster scanning [7]. Central subfield thickness (CST) is commonly used as an OCT parameter to evaluate the severity of DME and its prognosis. Previous studies, however, only reported a low to moderate correlation between CST and visual acuity (VA) [8]. This can be explained by two factors. First, there are significant individual differences, especially gender, in CST [9, 10]. Second, retinal atrophy leads to a reduction in CST but may exacerbate VA [11]. In fact, given the high resolution of OCT, researchers can obtain much more information than with CST. The outline of the cysts in the retina is clearly displayed in OCT images, which makes it possible to quantify the central macular fluid (CMF). Thus, the direct quantification of CMF rather than CST may better suggest the prognosis of anti-VEGF treatment of DME.
Recently, artificial intelligence (AI) based on deep learning (DL) has seen rapid development in ophthalmic image processing, especially for OCT images [12]. Several studies have quantified CMF using AI-assisted methods. You et al. [13] suggested that central macular fluid volume (CMFV) is a more accurate diagnostic biomarker than CST for DME. Tsuboi et al. [14] reported that the CMFV in the inner nuclear layer (INL) had a stronger association with VA than CST in DME before anti-VEGF treatment. However, no studies have compared the accuracy of CMFV and CST as prognostic factors for the anti-VEGF treatment of DME. Based on the proven effect of DME-associated fluid on visual function, we hypothesized that CMFV is a more accurate prognostic factor than CST for the anti-VEGF treatment of DME.
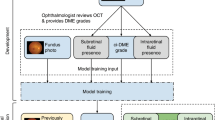
The purpose of this study was to develop two DL models to automatically measure the CMFV and the CST on the OCT volumetric scans and then investigate whether CMFV may serve as a better prognostic factor than CST to predict treatment response to anti-VEGF treatment in patients with DME.
Methods
Study Design, Setting, and Selection Criteria
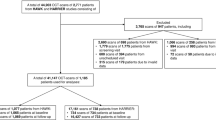
This study received approval from the Ethics Committee of Zhejiang Provincial People’s Hospital (KY2022063) and Eye Hospital of Wenzhou Medical University (H2023-005-K-03-01). The study was carried out in accordance with the Declaration of Helsinki of 1964 and its later amendments. Informed consent to publish was obtained from all participants before their inclusion in the study. The study included consecutive patients diagnosed with DME who received anti-VEGF treatment at the two medical centers from March 1, 2020, to December 31, 2022. DME was clinically diagnosed by a retina specialist based on the results of fundus examination, OCT, and angiography. The correlation of visual acuity with CMFV and CST was analyzed. A comparison of CMFV and CST at M0 for predicting best-corrected visual acuity (BCVA) ≥ 20/40 at M1 was calculated.
The inclusion criteria were as follows: (1) DME diagnosed by OCT; (2) eyes that were treatment-naïve or received retinal laser treatment more than 6 months earlier; (3) patients who received anti-VEGF therapy with aflibercept 2.0 mg/injection, conbercept 0.5 mg/injection, or ranibizumab 0.5 mg/injection during the study period; and (4) patients with available BCVA and OCT data at M0 and M1. Exclusion criteria included the following: eyes with any prior intravitreal pharmacotherapy, significant cataract, vitreous surgery, or other conditions that might influence visual acuity, such as corneal opacity, glaucoma, macular degeneration, retinal vein occlusion, and other retinal pathologies.
Clinical and Anatomical Data: Data Management and Assessments
At baseline (M0) and 1 month (M1) after anti-VEGF injection, patients underwent a comprehensive ocular examination before the surgery, including a dilated fundus examination, lens status, axial length measurement, spectral-domain OCT (SD-OCT) scans (Spectralis HD-OCT; Heidelberg Engineering, Heidelberg, Germany), and BCVA testing using a Snellen chart. The OCT volume scans were macula-centered, containing 19–49 B-scans and covering an area of 6.0 × 4.5 mm (20° × 15°). The OCT images of patients were transferred post hoc in a pseudonymized format for the AI-based analysis.
AI-Based CST Analysis Using OCT Images
All the study participants underwent thickness analysis using SD-OCT horizontal scans closest to the fovea. The CST measurements were defined as the average thickness within the central 1 mm-diameter circle centered on the fovea, from the inner border of the internal limiting membrane to the outer border of the retinal pigment epithelium (RPE). The CST was measured by a DL model. For the horizontal scan, the middle frame and two frames before and after were selected. After that, for each frame, the white pixel values of the segmentation results were counted from top to bottom from half of the width and two pixels to the left and right of each frame. The calculated pixel values were further transformed into standard units of micrometers, according to the scale of the original image, and the results for each position of a single frame were averaged. Finally, the results of multiple frames were averaged again for the final measurement.
AI-Based CMFV Analysis Using OCT Images
Auto Scale Detection
The traditional calculation of the volume of CMF relies on parameters such as pixel scale and scanning step provided by the OCT system. However, these parameters can differ from the system values with different patients and different scanning modes. Therefore, we designed an automatic scale detection method based on pixel feature matching, referred to as the CMFV intelligent quantification (CMFVIQ) model. According to the pixel characteristics of OCT scanning and imaging images, the scanning step (\(stepL\)) and the pixel scale \((scale\_w, scale\_h\)) in the horizontal and vertical directions can be detected quickly and accurately (as shown in Fig. 1).
CMF Segmentation
The purpose of segmentation was to calculate the area of the CMF region in each OCT slice. The CMF segmentation model was trained using U-Net, which included a contraction path for capturing context and a symmetric expansion path for precise localization. The model output a probability map with the same resolution as the input image, representing the probability that each pixel belonged to a CMF. Training was performed on a large collection of clinical datasets. Data annotation was performed according to reference standards designed by ophthalmologists with more than 10 years of clinical experience.
Volume Computation
The volume of the CMF was calculated using the integral method, and the calculation formula is as follows:
where \({\mathrm{area}}_{i}\) represents the CMF area of the \({i}_{th}\) OCT image, \(n\) represents the number of the OCT image sequence, and \({d}_{h}\) represents the scanning step (\(stepL)\).
The CMF area of an OCT image was calculated using the following formula:
where \({\mathrm{area}}_{\mathrm{pixel}}\) represents the area of a pixel, and \({N}_{\mathrm{DME}}\) is the sum of pixels occupied by the CMF.
Statistical Analysis
Statistical analysis was performed with SPSS Version 18 (SPSS Inc., Chicago, IL, USA). The distributions of the features were non-normal, as assessed using the Shapiro–Wilk test. Non-parametric tests were therefore used for statistical analysis. The Wilcoxon signed-rank test was used to compare CMFV, CST, and logarithm of the minimum angle of resolution (logMAR) BCVA between eyes at M0 and M1. Univariate and multivariate regression analysis were used to examine the correlation of visual acuity with CMFV and CST. The area under the receiver operating characteristic curve (AUROC) of CMFV and CST for predicting BCVA ≥ 20/40 at M1 was calculated. Statistical significance was set at p < 0.05.
Results
Demographics and Baseline Characteristics
We included 7305 OCT B-scans of 156 eyes from 89 patients with DME. The baseline characteristics of the subjects are presented in Table 1. Of the 156 eyes, 93 were from men and 63 were from women. The median age of the participants at baseline was 59 (50–67) years. The median glucose was 9.64 (6.31–11.64) mmol/L, while the median HbA1c was 7.46 (6.26–8.77)%. At baseline, 152 eyes (97.43%) had intraretinal fluid, and 60 eyes (26.92%) had subretinal fluid. A total of 13 (8.33%) eyes were in the aflibercept group, 62 (39.74%) eyes were in the conbercept group, and 81 (51.92%) eyes were in the ranibizumab group.
Evaluation Performance of the AI System
The performance of the CMFVIQ model is shown in Table 2 and Fig. 2. The sensitivity and specificity of the model reached 0.84 and 0.99, respectively. The Dice coefficient between the prediction and the ground truth marked by experts reached 0.72. These results support the effectiveness of the model for CMF segmentation. Figure 2 shows the CMF segmentation results between the AI-based DME segmentation model and human manually marked ground truth.
AI-Based Analysis of the Anti-VEGF Treatment Outcomes
CMFV, CST, and logMAR BCVA at baseline and M1 are listed in Table 3. At M0, the median of the CMFV was 0.272 (0.061–0.568) mm3, and that of the CST was 414 (293–575) μm. After the first anti-VEGF treatment, the CMFV decreased to 0.096 (0.018–0.307) mm3, and the CST decreased to 322 (252–430) μm, both of which were significantly different from the baseline (p < 0.001). The median of logMAR BCVA was 0.523 (0.301–0.817). After the first anti-VEGF treatment, the logMAR BCVA decreased to 0.398 (0.222–0.699), which was significantly different from the baseline (p < 0.001). All anti-VEGF agents influenced the reduction of CMFV, CST, and logMAR BCVA (see Supplementary Material). We further analyzed the differences in the effect of the three anti-VEGF agents on the change in CMFV, CST, and logMAR BCVA. However, there was no difference among the three anti-VEGF agents (see Supplementary Material).
Correlation of OCT Parameters and Visual Acuity at 1 Month
The association between CMFV and logMAR BCVA at baseline was firstly analyzed. The univariate regression analysis demonstrated that the logMAR BCVA at baseline correlated significantly with the CMFV (r = 0.315; 95% CI 0.164–0.466, p < 0.001) (Fig. 3). There was also a significant correlation between CST and logMAR BCVA at baseline (r = 0.309; 95% CI 0.158–0.460, p < 0.001), which was lower than the correlation between CMFV and logMAR BCVA at baseline. According to the multivariate analysis, the logMAR BCVA at baseline still correlated significantly with the CMFV (standard β = 0.199, p = 0.047), but was not correlated significantly with the CST (p = 0.071) (Table 4).
The association between CMFV at baseline and logMAR BCVA at M1 was further analyzed. The univariate regression analysis demonstrated that the logMAR BCVA at M1 correlated significantly with the CMFV (r = 0.385; 95% CI 0.238–0.532, p < 0.001) (Fig. 3). There was also a significant correlation between CST at baseline and logMAR BCVA at M1 (r = 0.344; 95% CI 0.195–0.494, p < 0.001), which was lower than the correlation between CMFV at baseline and logMAR BCVA at M1. The multivariate analysis revealed that the logMAR BCVA at M1 still correlated significantly with the CMFV (standard β = 0.279, p = 0.004), but was not correlated significantly with the CST (p = 0.091) (Table 5).
In addition, the prediction accuracy of the BCVA ≥ 20/40 for CMFV and CST was analyzed. The AUROC of CMFV for predicting eyes with BCVA ≥ 20/40 was 0.72 (p < 0.001), which was larger than that of CST at 0.69 (p < 0.001) (Fig. 4); however the difference was not significant (p = 0.360).
Discussion
In this study, we developed two DL models for quantifying CMFV and CST in patients with DME, and analyzed the relationship between CMFV and BCVA. The results showed that the relationship between CMFV at M0 and BCVA at M1 was stronger than that between CST and BCVA in DME patients. Previous studies have indicated that CST is the essential biomarker for the treatment and prognosis of DME [15]. Most countries and regions still use the changes in CST to evaluate the progression of DME in patients during clinical treatment [16, 17]. However, CST is influenced by a variety of factors in addition to the severity of the DME, and may not be sufficiently accurate to be used as a prognostic factor in clinical practice.
Because of the high resolution of OCT for observing retinal morphology, researchers are seeking a valid OCT parameter to use in the monitoring of DME in clinical practice. Doctors and researchers have used CST to evaluate the severity of DME and the efficacy of anti-VEGF therapy. However, CST measurement is easily affected by many factors, such as age, sex, axial length, and retinal atrophy [18]. Moreover, mixed results regarding the relationship between CST and postoperative vision have been reported. For example, Zhang et al. used machine learning to predict the vision of DME patients after anti-VEGF treatment and found that CST was an important predictive factor for VA prognosis [19], whereas Bressler et al. found that there was no strong correlation between baseline CST and long-term vision results [8]. CST, therefore, may not be an ideal biomarker for the prediction of outcomes of anti-VEGF treatment for DME patients.
Recent studies have demonstrated a qualitative relationship between CMFV and vision in patients with DME after anti-VEGF surgery, suggesting that CMFV may be a more specific indicator of DME diagnosis and disease change [20]. However, there is still a lack of means to accurately quantify retinal fluid in DME. With the development of DL, combined with OCT images to objectively identify and quantify retinal fluid, a new form of disease management can be realized for retinal exudative diseases. Liefers et al. developed a model which was applied to identify follow-up OCT volumes in patients who received anti-VEGF treatment to quantity OCT morphological parameters. However, precise segmentation and quantification of retinal fluid represent a challenge. First, the variability in retinal fluid directly increases the difficulty of segmentation. Second, it is hard to obtain a large amount of marker data required for model training, and the stability of manual segmentation is poor, making this task extremely tedious. The current public dataset of DME OCT images is insufficient to train a model to accurately segment and quantify retinal fluid [21]. Third, the motion artifacts and low signal-to-noise ratio result in a significant decline in OCT image quality [22]. Finally, the actual pixel scale and scan step parameters of OCT images differ among OCT models based on different scan modes and patients, which makes it difficult to accurately quantify retinal fluid by OCT model parameter values alone.
The proposed CMFVIQ model based on DL achieved an automatic and accurate measurement of CMFV. The automatic scale detection module actualized the automatic calculation of pixel scale and scan step size for OCT images of different scanning modes and patients. The CMF segmentation model was trained based on a large amount of collected OCT data. The model, robust to interference such as motion artifacts and low signal-to-noise ratios caused by retinopathy, reached a Dice coefficient, sensitivity, and specificity of 0.72, 0.84 and 0.99. The CMFVIQ model is trained based on the classic medical segmentation network U-Net [23]. Its core idea is to combine the encoder part of the convolutional neural network (capturing context information) and the decoder part (capturing pixel-level detail information) to achieve high-resolution image segmentation. There are already many U-Net-based variant networks, such as Swin-Unet [24]. In the future, we will consider using these networks to further improve the effectiveness of CMF segmentation.
Our results demonstrate the clinical value of CMFV in the management of patients with DME. In this study, a significant correlation between CMFV and logMAR BCVA was found with both univariate and multivariate analysis. CST, however, correlated significantly only with logMAR BCVA in univariate analysis. In addition, the AUROC of CMFV for predicting eyes with BCVA ≥ 20/40 at M1 was higher than the AUROC of CST. Therefore, CMFV rather than CST could be used as a biomarker to monitor DME patients. The correlation between CMFV and logMAR BCVA was only moderate (r = 0.315 for M0; r = 0.385 for M1), which is consistent with the finding of You et al. [13]. This result is also understandable because CMFV mainly evaluates edema and misses the retinal pathological features of DME, such as structural disorganization or loss. The accurate segmentation and quantification of retinal fluid can help to monitor the state of disease and provide research approaches for precision medicine. Quek et al. [25] proposed a DL-based AI application which provides personalized, continuous monitoring for patients at risk of retinal fluid complications by quantifying the total fluid area in OCT B-scan images. In addition, CMFV quantification can alert patients and clinicians to any dangerous increase in fluid so that steps can be taken to prevent further deterioration and progression. At the same time, through retrospective analysis of the data, if the intervention measures do not control the total fluid over several anti-VEGF treatments, treatment can be stopped, thus saving resources and costs for patients.
This study still had some limitations. First, the short-term follow-up duration made it challenging to infer the relationship between CMFV and vision in continuous long-term treatment. Second, this study lacked multicenter generalization. Future studies will incorporate datasets from multiple clinical centers, multiple devices, and multiple retinal diseases to understand the clinical application of the model.
Conclusions
The AI-based measured CMFV was a more accurate prognostic factor than CST for the initial anti-VEGF treatment outcome for DME and may provide additional information for the formulation of an optimal individualized therapeutic regimen.
References
Zhang J, Zhang J, Zhang C, Zhang J, Gu L, Luo D, Qiu Q. Diabetic macular edema: current understanding molecular mechanisms and therapeutic implications. Cells. 2022;11(21):3362.
D. Control, C.T.R. Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968–83.
Mathur R, Bhaskaran K, Edwards E, Lee H, Chaturvedi N, Smeeth L, Douglas I. Population trends in the 10-year incidence and prevalence of diabetic retinopathy in the UK: a cohort study in the Clinical Practice Research Datalink 2004–2014. BMJ Open. 2017;7(2): e014444.
Maturi RK, Glassman AR, Josic K, Baker CW, Gerstenblith AT, Jampol LM, Meleth A, Martin DF, Melia M, Punjabi OS, Rofagha S, Salehi-Had H, Stockdale CR, Sun JK. Four-Year Visual Outcomes in the Protocol W Randomized Trial of Intravitreous Aflibercept for Prevention of Vision-Threatening Complications of Diabetic Retinopathy. JAMA. 2023;329(5):376–85.
Li Y-F, Ren Q, Sun C-H, Li L, Lian H-D, Sun R-X, Su X, Yu H. Efficacy and mechanism of anti-vascular endothelial growth factor drugs for diabetic macular edema patients. World J Diabetes. 2022;13(7):532–42.
Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW, Bressler NM, Elman MJ, Ferris FL, Gardner TW, Jampol LM, Martin DF, Melia M, Stockdale CR, Beck RW. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018;136(10):1138–48.
Brais-Brunet S, Heckel É, Kanniyappan U, Chemtob S, Boudoux C, Joyal J-S, Dehaes M. Morphometric and Microstructural Changes During Murine Retinal Development Characterized Using In Vivo Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2021;62(13):20.
Bressler NM, Odia I, Maguire M, Glassman AR, Jampol LM, MacCumber MW, Shah C, Rosberger D, Sun JK. Association Between Change in Visual Acuity and Change in Central Subfield Thickness During Treatment of Diabetic Macular Edema in Participants Randomized to Aflibercept Bevacizumab, or Ranibizumab: A Post Hoc Analysis of the Protocol T Randomized Clinical Trial. JAMA Ophthalmol. 2019;137(9):977–85.
Hashemi H, Khabazkhoob M, Yekta A, Emamian MH, Nabovati P, Fotouhi A. The Distribution of Macular Thickness and Its Determinants in a Healthy Population. Ophthalmic Epidemiol. 2017;24(5):323–31.
von Hanno T, Lade AC, Mathiesen EB, Peto T, Njølstad I, Bertelsen G. Macular thickness in healthy eyes of adults (N = 4508) and relation to sex, age and refraction: the Tromsø Eye Study (2007–2008). Acta Ophthalmol. 2017;95(3):262–9.
Virgili G, Menchini F, Casazza G, Hogg R, Das RR, Wang X, Michelessi M. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev. 2015;1:CD008081.
Ting DSW, Pasquale LR, Peng L, Campbell JP, Lee AY, Raman R, Tan GSW, Schmetterer L, Keane PA, Wong TY. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167–75.
You QS, Tsuboi K, Guo Y, Wang J, Flaxel CJ, Bailey ST, Huang D, Jia Y, Hwang TS. Comparison of Central Macular Fluid Volume With Central Subfield Thickness in Patients With Diabetic Macular Edema Using Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2021;139(7):734–41.
Tsuboi K, You QS, Guo Y, Wang J, Flaxel CJ, Bailey ST, Huang D, Jia Y, Hwang TS. Association Between Fluid Volume in Inner Nuclear Layer and Visual Acuity in Diabetic Macular Edema. Am J Ophthalmol. 2022;237:164–72.
Patrao NV, Antao S, Egan C, Omar A, Hamilton R, Hykin PG, Sivaprasad S, Rajendram R. Real-World Outcomes of Ranibizumab Treatment for Diabetic Macular Edema in a United Kingdom National Health Service Setting. Am J Ophthalmol. 2016;172:51–7.
Hodzic-Hadzibegovic D, Sander BA, Monberg TJ, Larsen M, Lund-Andersen H. Diabetic macular oedema treated with intravitreal anti-vascular endothelial growth factor - 2–4 years follow-up of visual acuity and retinal thickness in 566 patients following Danish national guidelines. Acta Ophthalmol. 2018;96(3):267–78.
Reddy RK, Pieramici DJ, Gune S, Ghanekar A, Lu N, Quezada-Ruiz C, Baumal CR. Efficacy of Ranibizumab in Eyes with Diabetic Macular Edema and Macular Nonperfusion in RIDE and RISE. Ophthalmology. 2018;125(10):1568–74.
Torjani A, Mahmoudzadeh R, Salabati M, Cai L, Hsu J, Garg S, Ho AC, Yonekawa Y, Kuriyan AE, Starr MR. Factors Associated with Fluctuations in Central Subfield Thickness in Patients with Diabetic Macular Edema Using Diabetic Retinopathy Clinical Research Protocols T and V. Ophthalmology Science. 2023;3(1): 100226.
Zhang Y, Xu F, Lin Z, Wang J, Huang C, Wei M, Zhai W, Li J. Prediction of Visual Acuity after anti-VEGF Therapy in Diabetic Macular Edema by Machine Learning. J Diabetes Res. 2022;2022:5779210.
You QS, Tsuboi K, Guo Y, Wang J, Hwang TS. Comparison of Central Macular Fluid Volume With Central Subfield Thickness in Patients With Diabetic Macular Edema Using Optical Coherence Tomography Angiography. JAMA Ophthalmology. 2021;139(7):734–41.
Khan SM, Liu X, Nath S, Korot E, Faes L, Wagner SK, Keane PA, Sebire NJ, Burton MJ, Denniston AK. A global review of publicly available datasets for ophthalmological imaging: barriers to access, usability, and generalisability. Lancet Digit Health. 2021;3(1):e51–66.
Enders C, Lang GE, Dreyhaupt J, Loidl M, Lang GK, Werner JU. Quantity and quality of image artifacts in optical coherence tomography angiography. PLoS ONE. 2019;14(1): e0210505.
Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. Cham: Springer International Publishing; 2015. p. 234–41.
Cao H, Wang Y, Chen J, Jiang D, Zhang X, Tian Q, Wang M. Swin-Unet: Unet-Like Pure Transformer for Medical Image Segmentation. Cham: Springer Nature Switzerland; 2023. p. 205–18.
Quek TC, Takahashi K, Kang HG, Thakur S, Deshmukh M, Tseng RMWW, Nguyen H, Tham Y-C, Rim TH, Kim SS, Yanagi Y, Liew G, Cheng C-Y. Predictive, preventive, and personalized management of retinal fluid via computer-aided detection app for optical coherence tomography scans. EPMA J. 2022;13(4):547–60.
Acknowledgements
Funding
This work was supported by the Zhejiang Medical and Health Science and Technology Plan Project (2023KY915). The sponsor or funding organization had no role in the design or conduct of this research. The Zhejiang Provincial People’s Hospital funded the journal’s Rapid Service fee.
Author Contributions
Study concept and design: Xin Ye, Lijun Shen. Acquisition, analysis, or interpretation of data: Xin Ye, Kun Gao, Shucheng He, Xiaxing Zhong, Yingjiao Shen. Drafting of the manuscript: Xin Ye, Kun Gao, Shucheng He. Critical revision of the manuscript for important intellectual content: Lijun Shen. Final approval of the version to be published: Xin Ye, Lijun Shen. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Xin Ye, Lijun Shen.
Disclosures
Xin Ye, Kun Gao, Shucheng He, Xiaxing Zhong, Yingjiao Shen, Yaqi Wang, Hang Shao, and Lijun Shen have nothing relevant to disclose.
Compliance with Ethics Guidelines
This research received approval from the Ethics Committee of Zhejiang Provincial People’s Hospital (KY2022063) and Eye Hospital of Wenzhou Medical University (H2023-005-K-03–01). The study was carried out in accordance with the Declaration of Helsinki of 1964 and its later amendments. Informed consent to publish was obtained from all participants before their inclusion in the study.
Data Availability
These data cannot be made publicly available due to privacy concerns and a lack of informed consent for data sharing. Data are available from the Zhejiang Provincial People’s Hospital and Eye Hospital of Wenzhou Medical University for researchers who meet the criteria for access to confidential data.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ye, X., Gao, K., He, S. et al. Artificial Intelligence-Based Quantification of Central Macular Fluid Volume and VA Prediction for Diabetic Macular Edema Using OCT Images. Ophthalmol Ther 12, 2441–2452 (2023). https://doi.org/10.1007/s40123-023-00746-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00746-5