Abstract
Introduction
Cystoid macular edema (CME) is the most common cause of central vision loss in eyes with branch retinal vein occlusion (BRVO eyes). In recent literature, choroidal vascularity index (CVI) has been proposed to be an enhanced depth imaging optical coherence tomography (EDI-OCT) metric that may help characterize choroidal vascular changes in the setting of retinal ischemia, and potentially prognose visual outcomes and treatment patterns for patients with BRVO-related CME. This study sought to further characterize choroidal vascular changes in BRVO by comparing the CVI, subfoveal choroidal thickness (SFCT), and central subfield thickness (CST) in BRVO eyes with CME compared to unaffected fellow eyes.
Methods
This was a retrospective cohort study. Subjects included treatment-naïve BRVO eyes with CME diagnosed within 3 months of onset of symptoms and unaffected fellow eyes. EDI-OCT images were collected at baseline and at the 12-month follow-up visit. CVI, SFCT, and CST were measured. Demographics, treatment patterns, and best-corrected visual acuity (VA) were abstracted. Median CVI, SFCT, CST, and VA were compared between the two cohorts. Longitudinal relationships between these variables were analyzed.
Results
A total of 52 treatment-naïve eyes with BRVO and CME and 48 unaffected fellow eyes were identified. Baseline CVI was lower in eyes with BRVO than in fellow eyes (64.7% vs. 66.4%, P = 0.003). At 12 months, there was no difference in CVI between BRVO eyes and fellow eyes (65.7% vs 65.8%, P = 0.536). In BRVO eyes, there was a strong correlation between reduced CST and improved VA over the 12-month study period (r = 0.671, P < 0.001).
Conclusion
There are differences in CVI in treatment-naïve BRVO eyes with CME at presentation compared to fellow eyes, but these differences resolve over time. Anatomic changes in macular thickness in BRVO eyes with CME may be correlated with VA outcomes.
Plain Language Summary
Our study evaluated a novel ocular optical coherence tomography imaging metric, the choroidal vascularity index, in eyes that developed cystoid macular edema, a condition which can significantly impair acuity of central vision, after being diagnosed with branch retinal vein occlusion. In each patient, we compared the choroidal vascularity index in eyes that developed treatment-naïve, newly diagnosed branch retinal vein occlusion with cystoid macular edema to the non-diseased fellow eye. We made comparisons at the time of diagnosis (baseline) and at the 12-month follow up, and analyzed changes over time. We found that at the baseline visit, branch retinal vein occlusion eyes with cystoid macular edema had a significantly lower choroidal vascularity index than their unaffected fellow eyes, but that the differences between eyes resolved by the 12-month follow-up visit. Our findings suggest that choroidal vascularity may be compromised in the acute phase of branch retinal vein occlusion, but that this phenomenon resolves over time. Future research should further evaluate whether imaging characteristics of choroidal vascularity may be associated with changes in anatomic and visual outcomes in retinal diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? | |
Cystoid macular edema (CME) is the most common cause of central vision loss in eyes with branch retinal vein occlusion (BRVO), and novel ocular imaging metrics, such as the choroidal vascularity index (CVI), may help characterize choroidal vascular changes in BRVO eyes and potentially prognose visual outcomes and treatment patterns for patients with BRVO-related CME. | |
This study sought to further characterize choroidal vascular changes in BRVO by comparing CVI, subfoveal choroidal thickness (SFCT), and central subfield thickness (CST) in BRVO eyes with CME compared to unaffected fellow eyes from the time of BRVO diagnosis (baseline visit) to the 12-month follow-up visit. | |
What was learned from the study? | |
We found that at baseline visit, BRVO eyes with CME had significantly lower CVI than their unaffected fellow eyes, but that the differences between eyes resolved by the 12-month follow-up visit. | |
Our findings suggest that choroidal vascularity may be compromised in the acute phase of BRVO, but that this phenomenon resolves over time. | |
Future research should further evaluate whether imaging characteristics of choroidal vascularity may be associated with changes in anatomic and visual outcomes in retinal diseases. |
Introduction
Branch retinal vein occlusion (BRVO) is the second most common retinal vascular disease, estimated to affect 14 million people worldwide [1]. Its pathogenesis is related to the interruption of venous flow at an arteriovenous crossing, overwhelming the retinal venous tree [2]. The resulting ischemia can lead to secretion of vascular endothelial growth factor (VEGF), which increases retinal vascular permeability and contributes to cystoid macular edema (CME) [3,4,5]. CME is the most common cause of vision loss in BRVO [6, 7], with studies citing BRVO-related CME in up to 97% of affected eyes [8, 9]. In a study by the Branch Vein Occlusion Study group, more than 60% of eyes with BRVO-related CME had best-corrected visual acuity (VA) worse than 20/40 without treatment [10]. Understanding the pathophysiology of CME in eyes with BRVO (referred to further as BRVO eyes) may lead to better treatment decision-making and improve visual prognosis for patients with BRVO-related CME.
Enhanced depth imaging optical coherence tomography (EDI-OCT) has emerged as an imaging method that facilitates a better understanding of the changes in choroidal vasculature in BRVO [11]. Prior studies have used EDI-OCT to assess subfoveal choroidal thickness (SFCT), which is typically greater in eyes with acute BRVO compared to unaffected fellow eyes [12, 13]. However, the correlation between SFCT and visual outcomes in BRVO eyes following treatment with intravitreal anti-VEGF injections is unclear [14,15,16]. Many prior studies only evaluated clinical outcomes within 3 months of initial presentation, and such short-term results may not translate to more long-term clinical outcomes for BRVO. Furthermore, while SFCT measurement provides an estimation for overall choroidal volume, SFCT reflects the total choroidal surface and does not specifically describe choroidal angioarchitecture.
In recent literature, the choroidal vascularity index (CVI) has been proposed as a metric that may better represent choroidal vascularity integrity than SFCT alone. The CVI is defined as the ratio between vascular luminal area (LA) and total choroidal area (TCA), which is the sum of LA and choroidal stromal area (SA) on EDI-OCT [17, 18]. Having been studied in other retinal and chorioretinal conditions as a potentially more robust metric of choroidal vascular integrity [19,20,21], CVI is now being studied in BRVO. Prior studies have identified a lower CVI in BRVO eyes compared to unaffected fellow eyes and healthy control eyes [22,23,24]. However, data from investigations on the longitudinal relationship between CVI and macular thickness as an outcome metric for CME are limited.
We present here a retrospective cohort study in BRVO eyes to investigate the relationship between CVI, SFCT, and central subfield thickness (CST) in eyes with BRVO-related CME.
Methods
Subject Recruitment and Inclusion Criteria
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Ethical approval for this study was obtained from the Duke University Health System Institutional Review Board (IRB # Pro00075701). Informed consent was not sought for the present study because of the retrospective nature of the study involving several data points and images from each patient record which were deidentified prior to data analysis. The IRB authorized retrospective chart review. Subjects in the Duke Retinal Vein Occlusion (RVO) Database were studied. All subjects were aged ≥ 18 years and had presented for evaluation of treatment-naïve unilateral BRVO at the Duke Eye Center between January 2009 and January 2021. Patients were identified using the Duke Enterprise Data Unified Content Explorer (DEDUCE) search tool. Onset of symptoms occurred within 3 months before initial evaluation at Duke, and all participants had at least 12 ± 3 months of follow-up. All subjects had a dilated fundus examination documenting macula-involving BRVO with CME and EDI-OCT of at least the BRVO eye at baseline and at 12 months. In this study population, CME was defined as any hyporeflective cystic space in the retina felt to represent fluid on macular OCT.
Any fellow eye with a history of BRVO or a new diagnosis of BRVO during the study period was excluded. Subjects were also excluded if they had prior vitreoretinal surgery, intravitreal injections, CME, choroidal neovascularization, diabetic retinopathy, central serous chorioretinopathy, pachychoroid syndromes, or myopic macular degeneration.
Design
This was a retrospective cohort study. Eyes were separated into the following cohorts: (1) eyes with treatment-naïve BRVO and CME and (2) unaffected fellow eyes.
Image Collection and CVI Measurement
The SPECTRALIS® ophthalmic imaging platform (Heidelberg Engineering GmbH, Heidelberg, Germany) with the manufacturer’s Spectralis software version 1.0.16.0 was used to collect all macular OCT images, which were horizontal 30°, 9-mm high-speed line A-scans with an automated real time of up to 100. The Heidelberg software uses automated eye tracking during image acquisition, and for each image for each eye, the OCT section centered directly on the fovea was selected for analysis. The Heidelberg software measured SFCT and CST for all OCT images. SFCT was measured using a caliper tool to draw a line perpendicular to the hyperreflective outer border of the retinal pigment epithelium to the hyperreflective sclerochoroidal junction by two trained graders (PP, RA). Measurements for CST, an objective parameter to estimate macular thickness and approximate amount of CME [25], representing the average thickness of all points lying within the 1-mm central subfield as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) [26], were automatically generated by the Heidelberg software.
The COIN portal (www.ocularimaging.net [27]), a collaborative portal for ocular image analysis designed by one of the co-authors (RA), was used to generate CVI measurements. All de-identified EDI-OCT images were uploaded to the COIN portal. The choroid region of interest (ROI), which was a 1.0-mm horizontal section of subfoveal choroid centered on the fovea, was identified semi-automatically using the inbuilt ROI selection tool. For this section, LA and TCA were measured using an automated inbuilt image binarization tool on COIN, and CVI (LA/TCA) was generated as an output of this analysis. LA and TCA were measured in a masked fashion in both eyes at baseline and at 12 months. The choroid ROI selection and CVI measurements performed by a trained medical student grader (PP) were reviewed for accuracy by an expert attending physician grader from the COIN team (RA) (Fig. 1). Unfavorable artifacts were addressed using automated and manual correction techniques. All OCT images were first reviewed by the two graders (PP, RA), and any that were deemed to be of poor quality based on resolution or presence of artifacts obscuring the macula and/or subfoveal choroid were excluded. During grading, the COIN system automatically corrected or ignored artifacts by adjusting the brightness and contrast of the image, removing noise, or smoothing the image. If there were too many artifacts in a particular B-scan image, that image was ignored and not taken for analysis based on the grader’s judgment. Lastly, by manual adjustment, the ROI for the image with fewest artifacts was constructed in a manner to eliminate the artifacts from the final ROI.
Illustration of the Comprehensive Ocular Imaging Network (COIN) console for computing the choroidal vascularity index (CVI) in eyes with macular edema. a Unsegmented original optical coherence tomography (OCT) scan. b The region of interest (ROI) created by the grader with help of automation for ROI wherein the retinal pigment epithelium–Bruch's membrane (RPE-Bruch) complex (upper limit of ROI) and choroidal scleral interface boundary (lower limit of ROI) can be manually adjusted at the grader’s discretion based on case-by-case assessment of the OCT scan. c Automated thresholding and binarization of the ROI to give the demarcation between luminal areas (LA) and stromal areas (SA) within the segmented choroid (ROI). d The reader panel of the console presenting the different values generated by the COIN platform after the image is binarized. It gives the option to fix the scale for conversion of pixels to microns and provides values of choroidal thickness (CT), CVI, total choroidal area (TCA), luminal area (LA), and stromal area (SA) for the left, mid, and right segments of the choroid. It also provides the grader with the ability to alter the span of the ROI and option of 1-click ACN (approve, collect results, and move to next image) to facilitate faster image analysis
Additional Data Collection
Data were also collected on patient demographics and best-corrected ETDRS VA as well as the total number and type of treatments for BRVO with CME over the 12-month study period.
Statistical Analysis
Data analysis for this paper was generated using SAS/STAT software, version 9.4 of the SAS System for Windows (SAS Institute, Cary, NC, USA). Differences between BRVO eyes and unaffected fellow eyes in clinical variables (CVI, SFCT, CST, VA) at both baseline and the 12-month follow-up visits were assessed using the Wilcoxon signed rank test. Changes from baseline to 12 months were compared between and within eyes using the Wilcoxon signed rank test. Spearman correlations of change from baseline in the four variables were computed. Graphics were also produced to assist in assessment of relationships between variables with respect to change.
Results
Demographics and Treatment Patterns
A total of 52 eyes diagnosed with treatment-naïve BRVO with CME that had EDI-OCT at baseline and at 12 months were identified, and 48 unaffected fellow eyes met the inclusion criteria (4 of the 52 fellow eyes did not undergo OCT imaging). Demographic data for all 52 patients are given in Table 1. The mean (± standard deviation) duration of BRVO prior to diagnosis at the Duke Eye Center was 1.2 ± 0.9 months. Forty-five of the BRVO eyes (86.5%) received at least one treatment for CME due to BRVO over the study period, including intravitreal bevacizumab (40.4%) (Avastin; Genentech, South San Francisco, CA, USA), ranibizumab (26.9%) (Lucentis; Genentech), aflibercept (51.9%) (Eylea; Regeneron, Tarrytown, NY, USA), triamcinolone acetonide (17.3%) (Triesence; Alcon, Geneva, Switzerland), grid-pattern laser photocoagulation (5.8%), or dexamethasone intravitreal implant (1.9%) (Ozurdex; Allergan, Irvine, CA, USA). Over the 12-month follow-up period, BRVO eyes received a mean of 7.2 ± 4.2 injections.
Comparisons of BRVO Eyes Versus Unaffected Fellow Eyes at Baseline and 12-Month Follow-up
At baseline visit for BRVO diagnosis (Table 2), CVI was lower in treatment-naïve BRVO eyes than in fellow eyes, but CST was higher in BRVO-affected eyes when compared to fellow eyes. VA was lower in BRVO eyes than in fellow eyes. There was no difference in SFCT between BRVO and fellow eyes.
At the 12-month follow-up visit (Table 3), following a mean of 7.2 ± 4.2 treatments, CST remained higher in BRVO-affected eyes than in fellow eyes, and VA remained lower in BRVO-affected eyes than in fellow eyes. However, there was no significant difference in CVI or SFCT between the cohorts.
Changes in BRVO-Related Characteristics Between Baseline and 12 Months
Over the study period, CST decreased while VA increased in the BRVO eyes (Table 4). At 12 months, there was an increase in CVI in the BRVO eyes, but the change was not significant. There was no change in SFCT in BRVO eyes. In fellow eyes, there were no changes in CVI, CST, SFCT, or VA over the study period.
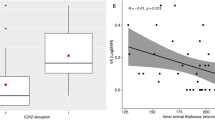
Correlations were assessed between changes in CVI, CST, SFCT, and VA over the study period in BRVO eyes (Table 5). There was no significant correlation between change in CVI and change in CST, SFCT, or VA over the study period. There was also no correlation between change in SFCT and change in CST or VA. However, there was a positive correlation between change in CST and change in logMAR VA over the study period, where a larger decrease in CST was associated with a greater improvement in VA (i.e., more letters gained on the ETDRS chart) (Fig. 2).
In BRVO eyes, there was no correlation between baseline CVI and change in CST or VA at 12 months. There was a weak negative correlation between baseline CVI and the number of treatments at 12 months, but this was not statistically significant (r = − 0.213, P = 0.129).
Discussion
This study details a longitudinal analysis of CVI and SFCT in a cohort of BRVO eyes managed at locations in the southeastern USA. Our main pertinent positive finding was that baseline CVI was lower in treatment-naïve BRVO eyes with CME than in unaffected fellow eyes but equalized within 12 months. We also found a strong correlation between change in CST and change in VA in BRVO eyes over the 12-month study period. We will also discuss pertinent negative findings regarding SFCT and longitudinal relationships between CVI, SFCT, and CST in BRVO eyes with CME.
This study’s key finding was that baseline CVI was lower in BRVO eyes with CME than in the unaffected fellow eyes, but this difference was no longer present at 12 months. While the increase in CVI in BRVO eyes over 12 months was not significant, there was no difference in CVI between BRVO and fellow eyes at 12 months, which suggests that CVI in BRVO eyes normalized over time following the acute insult. This also suggests that in the acute phase of BRVO, there may be decreased choroidal vascular LA relative to TCA in the affected eye with normalization of the choroidal vessel to total area ratio in BRVO eyes over time. Decreased baseline CVI in BRVO eyes is consistent with results reported from two prior studies [23, 24]. Meanwhile, Aribas et al. noted a lower CVI in both eyes of patients with BRVO compared to healthy control eyes, with a more robust decrease in BRVO eyes [22].
Authors of prior studies have proposed that lower CVI in BRVO eyes may be associated with an extracellular fluid shift from the retina to the choroid in the setting of retinal ischemia. Two studies reported an increase in choroidal SA in eyes with BRVO compared to fellow and control eyes, but no decrease in choroidal LA [22, 24]. These findings suggest that lower CVI represents an expansion of the choroidal SA rather than alterations to the choroidal vessels, supporting the theory that an extracellular fluid shift after the development of a BRVO causes choroidal stromal edema. The exact mechanism of this fluid shift is unclear. Given the association of CME in BRVO with overexpression of VEGF, it has been suggested in the literature that VEGF may also be linked to increased choroidal stromal edema in BRVO eyes [24, 28]. Alternatively, it has been theorized that this fluid shift may be due to an overexpression of aquaporin-9 between the retina and choroid in the setting of retinal ischemia [22]. Unique to our study is the finding that there was no difference in CVI between BRVO eyes and fellow eyes at the 12-month follow-up. This finding suggests that over time, the extracellular fluid shift in treated BRVO eyes decreases. Of note, more than 80% of the eyes we studied received anti-VEGF treatments during the study period. Given the normalization of CVI in BRVO eyes at 12 months, this may support the theory that the extracellular fluid shift in the acute BRVO phase may be due to overexpression of VEGF.
As CST decreased in BRVO eyes with CME longitudinally, the VA improved. CST is an objective parameter of macular thickness on spectral domain OCT (SD-OCT) and is used to quantify the degree of CME [26]. Other studies have referred to this parameter as central macular thickness (CMT), central subfield macular thickness (CSMT), and central retinal thickness (CRT) [25, 29]. Our finding associating decreased CST with improved VA is consistent with results from prior studies, but demonstrates a stronger correlation between reduced macular thickness measurements and improved VA over a longer follow-up period. Thach et al. identified a moderate correlation between reduced central foveal thickness (CFT) and improved VA at 6 months [6]. However, CFT was manually measured as the center point thickness—that is, the thickness from the innermost layer of the retinal inner nuclear layer to the hyperreflective outer border of the retinal pigment epithelium (RPE) [7]. CST is automatically generated by SD-OCT software and is a more reliable indicator of macular thickness than is center point thickness [25]. Ciulla et al. also demonstrated a similar but weaker correlation between changes in CST and changes in VA in 426 eyes with BRVO-related CME over 6 months [30]. We documented change over a 12-month period rather than a 6-month period, suggesting the relationship between macular thickness and VA may strengthen over time. It should be noted that prior studies have identified that reductions in macular thickness and improvements in VA in BRVO-affected eyes were not always simultaneous. In an analysis of eyes with BRVO-related CME treated with ranibizumab, some eyes with persistently higher CFT at 6 months had substantial improvements in VA, while some eyes with markedly reduced CFT had persistently poor VA [4, 6]. In the context of this prior literature, it is difficult to interpret how direct the correlation between reduced CST and improved VA is in our study. Additionally, other factors aside from changes in CST may affect VA outcomes in BRVO eyes, such as variability in underlying retinal ischemia and VA prior to onset of BRVO. Future investigations comparing the chronology of changes in CST compared to VA, rather than CFT, may elucidate whether CST can be used as a metric for long-term outcomes after acute BRVO-related CME.
This study was also notable for several negative findings. There was no difference in SFCT between BRVO eyes with CME and unaffected fellow eyes either at baseline or at the 12-month follow-up. There was no change in SFCT over the study period in either group. There was also no correlation between change in CVI and change in SFCT, CST or VA, and no correlation between change in SFCT and change in CST or VA over the study period in BRVO-affected eyes. There was also no correlation between baseline CVI and change in CST or VA in BRVO eyes.
Although eyes affected with BRVO typically have a higher SFCT than fellow or control eyes, some have found that SFCT can decrease after treatment with intravitreal anti-VEGF injections [13]. In studies of CVI and SFCT in BRVO eyes, Hwang et al. found no difference in SFCT between BRVO eyes and fellow eyes [23], while Alis and Alis found SFCT to be higher in BRVO eyes when compared to fellow eyes and control eyes of patients who did not have BRVO [24]. The reason for our findings is unclear. If an extracellular fluid shift causes choroidal stromal swelling in BRVO eyes, it is likely that choroidal thickness would also increase. It is possible that the dynamics of extracellular fluid shift may cause choroidal stroma to swell, lowering the LA/TCA ratio that defines CVI, while choroidal thickness may not necessarily change. It should be noted that CVI has been demonstrated to be a more repeatable measurement than SFCT, [31] which may in part explain discrepancies between SFCT and CVI findings.
Our assessment of correlations between CVI, SFCT, CST and VA is the third such analysis to date. Hwang et al. noted a weak correlation between CVI and SFCT in BRVO eyes with CME. Similar to our findings, Alis and Alis did not find any correlations between CVI, SFCT and VA in BRVO eyes, but they did not assess CST [24]. Although Hwang et al. did find a correlation between baseline CVI in fellow eyes and CMT in BRVO eyes with CME as well as a correlation between baseline CVI of fellow eyes and a 2-year change in VA in BRVO-affected eyes, we found no such correlations. Of note, Hwang et al. used swept-source OCT (SS-OCT), whereas SD-OCT was used in the present study; these two modalities are not directly comparable. Furthermore, Hwang et al. did not use the COIN platform for CVI measurements [23]. Further investigation is needed to determine the significance of these findings.
This retrospective study has some inherent limitations. First, while this study has a sample size comparable to or larger than most studies in existing literature studying CVI in BRVO eyes, it had a relatively small sample size of 52 BRVO eyes with CME and 48 unaffected fellow eyes. Second, four BRVO eyes did not have OCT images for the associated fellow eye. Third, eight eyes with BRVO-related CME did not receive any treatment over the 12-month study period; consequently, comparisons between treated and untreated eyes, or between types of treatment, were not made. Fourth, we were not able to compare CVI and SFCT between BRVO eyes that did and did not have CME, as the latter cohort was excluded due to small sample size.
Conclusion
This study aimed to further characterize choroidal vascular changes in patients with BRVO eyes and CME by comparing CVI, SFCT, and CST in affected eyes versus unaffected fellow eyes. The study was a retrospective cohort study that included treatment-naïve patients with BRVO and CME diagnosed within 3 months of symptom onset. The study found that at baseline, there was a significant difference in CVI between BRVO eyes with CME and unaffected fellow eyes, with the former having lower CVI values. However, this difference resolved by the 12-month follow-up visit, where there was no significant difference in CVI between the two groups. These findings suggest that CVI changes due to acute retinal insult in BRVO may resolve over time. The study also found a strong correlation between reduced CST and improved VA in BRVO eyes with CME over the 12-month study period. This highlights the importance of monitoring anatomic changes in macular thickness in BRVO eyes with CME as a potential predictor of VA outcomes.
Overall, the study provides important insights into the pathophysiology of BRVO and CME, and the role of CVI as a potential imaging biomarker for characterizing choroidal vascular changes in this population. The findings support the existing theory that VEGF plays a role in extracellular fluid shift in the choroid in the setting of acute BRVO. However, the study also highlights the need for further research to elucidate the pathophysiology of choroidal changes in BRVO and the relationship between molecular mediators of choroidal vascularity and imaging metrics. These findings have implications for the management and treatment of patients with BRVO and CME.
References
Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1094-101.e5.
Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008;33(2):111–31.
Kumar B, Yu DY, Morgan WH, Barry CJ, Constable IJ, McAllister IL. The distribution of angioarchitectural changes within the vicinity of the arteriovenous crossing in branch retinal vein occlusion. Ophthalmology. 1998;105(3):424–7.
Campochiaro PA. Anti-vascular endothelial growth factor treatment for retinal vein occlusions. Ophthalmologica. 2012;227(Suppl 1):30–5.
Noma H, Funatsu H, Yamasaki M, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005;140(2):256.e1-.e7.
Thach AB, Yau L, Hoang C, Tuomi L. Time to clinically significant visual acuity gains after ranibizumab treatment for retinal vein occlusion: BRAVO and CRUISE trials. Ophthalmology. 2014;121(5):1059–66.
Campochiaro PA, Heier JS, Feiner L,et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102-12.e1.
Takahashi MK, Hikichi T, Akiba J, Yoshida A, Trempe CL. Role of the vitreous and macular edema in branch retinal vein occlusion. Ophthalmic Surg Lasers. 1997;28(4):294–9.
Yamaguchi Y, Otani T, Kishi S. Serous macular detachment in branch retinal vein occlusion. Retina. 2006;26(9):1029–33.
The Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984;98(3):271–82.
Tsuiki E, Suzuma K, Ueki R, Maekawa Y, Kitaoka T. Enhanced depth imaging optical coherence tomography of the choroid in central retinal vein occlusion. Am J Ophthalmol. 2013;156(3):543-7.e1.
Rayess N, Rahimy E, Ying G-S, et al. Baseline choroidal thickness as a predictor for treatment outcomes in central retinal vein occlusion. Am J Ophthalmol. 2016;171:47–52.
Coban-Karatas M, Altan-Yaycioglu R, Ulas B, Sizmaz S, Canan H, Sariturk C. Choroidal thickness measurements with optical coherence tomography in branch retinal vein occlusion. Int J Ophthalmol. 2016;9(5):725–9.
Kim KH, Lee DH, Lee JJ, Park SW, Byon IS, Lee JE. Regional choroidal thickness changes in branch retinal vein occlusion with macular edema. Ophthalmologica. 2015;234(2):109–18.
Okamoto M, Yamashita M, Sakamoto T, Ogata N. Choroidal blood flow and thickness as predictors for response to anti-vascular endothelial growth factor therapy in macular edema secondary to branch retinal vein occlusion. Retina. 2018;38(3):550–8.
Tang F, Xu F, Zhong H, et al. Comparison of subfoveal choroidal thickness in eyes with CRVO and BRVO. BMC Ophthalmol. 2019;19(1):133.
Sonoda S, Sakamoto T, Yamashita T, et al. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol. 2015;159(6):1123-31.e1.
Agrawal R, Ding J, Sen P, et al. Exploring choroidal angioarchitecture in health and disease using choroidal vascularity index. Prog Retin Eye Res. 2020;77:100829.
Koh LHL, Agrawal R, Khandelwal N, Sai Charan L, Chhablani J. Choroidal vascular changes in age-related macular degeneration. Acta Ophthalmol. 2017;95(7):e597–601.
Agrawal R, Chhablani J, Tan K-A, Shah S, Sarvaiya C, Banker A. Choroidal vascularity index in central serous chorioretinopathy. Retina. 2016;36(9).
Wei X, Ting DSW, Ng WY, Khandelwal N, Agrawal R, Cheung CMG. Choroidal vascularity index: a novel optical coherence tomography based parameter in patients with exudative age-related macular degeneration. Retina. 2017;37(6).
Aribas YK, Hondur AM, Tezel TH. Choroidal vascularity index and choriocapillary changes in retinal vein occlusions. Graefes Arch Clin Exp Ophthalmol. 2020;258(11):2389–97.
Hwang BE, Kim M, Park YH. Role of the choroidal vascularity index in branch retinal vein occlusion (BRVO) with macular edema. PLoS One. 2021;16(10): e0258728.
Alis A, Guler AM. The effect of branch retinal vein occlusion on the vascular structure of the choroid. Photodiagn Photodyn Ther. 2022;37: 102687.
Network DRCR. Reproducibility of macular thickness and volume using zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. 2007;114(8):1520–5.
Early Treatment Diabetic Retinopathy Research Group. Early treatment diabetic retinopathy study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98(5 Suppl):741–56.
Betzler BK, Ding J, Wei X, et al. Choroidal vascularity index: a step towards software as a medical device. Br J Ophthalmol. 2022;106(2):149–55.
Chen L, Yuan M, Sun L, Chen Y. Three-dimensional analysis of choroidal vessels in the eyes of patients with unilateral BRVO. Front Med (Lausanne). 2022;9:854184.
Çekiç O, Çakır M, Yazıcı AT, Alagöz N, Bozkurt E, Faruk YÖ. A comparison of three different intravitreal treatment modalities of macular edema due to branch retinal vein occlusion. Curr Eye Res. 2010;35(10):925–9.
Ciulla TA, Kapik B, Grewal DS, Ip MS. Visual acuity in retinal vein occlusion, diabetic, and uveitic macular edema: central subfield thickness and ellipsoid zone analysis. Ophthalmol Retina. 2021;5(7):633–47.
Breher K, Terry L, Bower T, Wahl S. Choroidal biomarkers: a repeatability and topographical comparison of choroidal thickness and choroidal vascularity index in healthy eyes. Transl Vis Sci Technol. 2020;9(11):8.
Acknowledgements
Funding
This research was supported in part by the VitreoRetinal Surgery Foundation (Grant 388000009). The funding organization had no role in the design or conduct of this research. No funding or sponsorship was received for the publication of this article.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Praruj Pant, Anita Kundu, Jay Rathinavelu, Rupesh Agrawal, and Sandra S. Stinnett. The first draft of the manuscript was written by Praruj Pant, and all authors commented on all versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
No conflicting relationship or competing interest exists for any author.
Compliance with Ethics Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Ethical approval for this study was obtained from the Duke University Health System Institutional Review Board (IRB # Pro00075701). Informed consent was not sought for the present study because of the retrospective nature of the study involving several data points and images from each patient record which were deidentified prior to data analysis. The IRB authorized retrospective chart review.
Data Availability
All data generated or analyzed during this study is available as an electronic supplementary excel file published alongside the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pant, P., Kundu, A., Rathinavelu, J.K. et al. Longitudinal Assessment of the Choroidal Vascularity Index in Eyes with Branch Retinal Vein Occlusion-Associated Cystoid Macular Edema. Ophthalmol Ther 12, 2103–2115 (2023). https://doi.org/10.1007/s40123-023-00731-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00731-y