Abstract
Introduction
This study aimed to evaluate the changes that a recently developed at-home device using low-level light therapy (LLLT) produced in signs and symptoms of patients with dry eye disease (DED) owing to meibomian gland dysfunction (MGD).
Methods
In this prospective study, patients with DED owing to MGD not successfully responding to first-line therapy (tear substitutes and eye lid hygiene) were treated with four serial sessions (every other day) of mask based on LLLT technology and dedicated for home use (my-mask®, Espansione Marketing S.p.A., Bologna, Italy). Non-invasive ocular surface examination was carried out by means of Keratograph 5M (Oculus, Wetzlar, Germany) before and after four mask sessions for the evaluation of (i) tear meniscus height (TMH); (ii) first and average non-invasive Keratograph breakup time (NIKBUT); (iii) meibomian gland loss (MGL). Ocular Surface Disease Index (OSDI) questionnaire was used to assess ocular discomfort symptoms.
Results
Overall, 17 patients (3 male, 14 female; mean age 61.47 ± 11.93 years) were enrolled and all of them regularly completed the entire cycle of four sessions without reporting any adverse event. The mean values of NIKBUT first and NIKBUT average increased significantly after treatment (from 5.29 ± 2.60 at T0 to 9.04 ± 3.49 s at T1 [P = 0.001] and from 9.40 ± 3.81 to 11.28 ± 2.81 s [P = 0.017]); in parallel, the mean value of TMH increased significantly from 0.27 ± 0.06 to 0.32 ± 0.09 mm (P = 0.029). Conversely, there were not statistically significant differences for MGL (P = 0.346). In addition, the mean value of OSDI score decreased after treatment (from 32.00 ± 7.96 at T0 to 20.71 ± 8.03 at T1; P < 0.001).
Conclusions
One week of serial sessions of a newly developed LLLT device for home use significantly improved tear film production and stability along with ocular discomfort symptoms in patients with DED owing to MGD. These findings open up a new scenario for patients with MGD who can enjoy the unique benefits of LLLT at home.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Low-level light therapy (LLLT) is an emerging promising option for the treatment of dry eye disease (DED) owing to meibomian gland dysfunction (MGD) |
Previous studies have demonstrated the efficacy on patients’ signs and symptoms of in-office LLLT mask used alone or in combination with intense pulsed light in the setting of DED owing to MGD |
What was learned from the study? |
A weekly protocol of serial sessions with a newly developed LLLT mask for home use allowed the significant improvement of both tear production and stability along with the significant amelioration of ocular discomfort symptoms |
These results open up a new scenario for patients with MGD who can enjoy the unique benefits of LLLT technology at home |
Introduction
Dry eye (DED) is a multifactorial disease of the ocular surface [1]. Meibomian gland dysfunction (MGD) represents the most common cause of evaporative DED. Meibomian glands are located in the upper and lower eyelids and secrete meibum, a compound of polar and nonpolar lipids, forming the outermost layer of the tear film [2]. These lipids are responsible for the stability of the tear film and reduce ocular surface water evaporation [3]. In obstructive MGD, epithelial hyperkeratinization leads to duct obstruction, meibum stasis, cystic dilation, and, in the final stage, acinar atrophy, resulting in hyposecretion with subsequent tear film instability and increased evaporation [4,5,6,7]. These pathological processes determine the entry into the loop of a DED vicious circle causing a cascade of events characterized by inflammation and increased tear osmolarity [8, 9].
The traditional medical approach includes the daily use of tear substitutes combined with eyelid hygiene. In recalcitrant MGD cases, short courses of antibiotics or corticosteroids can be necessary to better control patients’ symptoms and signs.
In recent years, several device-assisted treatments have been developed for the treatment of MGD. These therapies can be classifiable according to the mechanism of action of the device in the following categories: eyelid warming; eyelid warming and massaging; light-based; massaging and light-based; electrotherapy; cleaning; tear production nerve stimulator [10,11,12,13,14,15].
Among them, the technology low-level light therapy (LLLT) is one of the most promising treatment options. This is a particular type of photo biomodulation, based on light-emitting diodes. It consists of sending low incident levels of photon energy, which is transferred directly to the absorbing cell or chromophore, causing photoactivation of the target cells. The photoactivated cells may better repair cellular damage and improve function and proliferation thanks to the increase adenosine triphosphate (ATP) production. At the level of the meibomian glands, this process could lead to endogenous heating, thus promoting the flow of meibum [16].
Several studies have demonstrated the efficacy of in-office LLLT used alone or in combination with intense pulsed light (IPL) in the setting of DED owing to MGD [17,18,19,20,21,22,23]. More recently, the same LLLT technology has been miniaturized into such a portable package (my-mask®) in order to provide a small yet powerful solution of light-based therapy for home use.
The aim of this study is to report the preliminary results of this novel LLLT-based mask used at home in patients with MGD.
Methods
Study and Patients
This prospective study included patients with MGD who presented for a routine visit to the ocular surface office of the University Magna Graecia (Catanzaro, Italy) between April 2022 and September 2022. The study was approved by the local ethics committee (Comitato Etico Regione Calabria, Sezione Area Centro, no. 189-2022) and followed the tenets of the Declaration of Helsinki for research involving human subjects. Written informed consent was obtained from all participants after the explanation of the nature of the study. Consecutive patients aged 18–80 years were screened for eligibility according to the following inclusion criteria: presence of MGD defined by the presence of signs consistent with gland terminal duct obstruction with abnormal quantity and/or quality of secretions; presence of at least one MGD-related ocular symptom among dryness, foreign body sensation, irritation, and burning; clinical picture not satisfactorily controlled despite the ongoing first-line therapy (tear substitutes and eyelid hygiene); pathological value of Ocular Surface Disease Index (OSDI) score (≥ 13); noninvasive breakup time (NIBUT) less than 10 s. Patients were excluded from the study if one or more of the following conditions was present: active eye inflammation; eyelid malposition; recent (within 3 months) ocular surgery (to avoid fluctuations of DED signs/symptoms that typically occur in the early postoperative phase); history of contact lens wearing; recent (within 1 month) usage of anti-inflammatory eye drops (topical corticosteroid or cyclosporine).
Ocular Surface Workup
All patients were examined before the first mask session (day 0, T0) and 1 day after the last session (day 7, T1) by means of Keratograph® 5M (Oculus, Germany) by an experienced examiner (S.V.). All the measurements were taken between 5:00 p.m. and 6:00 p.m. in one single visit in a dimly lit room with controlled temperature (21–24 °C) and humidity (30–60%). The workup included the evaluation of tear meniscus height (TMH), noninvasive Keratograph breakup time (NIKBUT), and infrared meibography. For each parameter, the measurements were repeated three times and the mean value was recorded and used for the statistical analysis. Briefly, TMH was measured along the lower lid margin immediately below the pupil. NIKBUT used Placido rings that were reflected on the corneal surface: the interval time between the last complete blinking and the first distortion the 22 concentric rings reflected on the corneal surface was defined as “NIKBUT first”; the average time of all tear film breakups occurring in the measured period of up to 24.98 s (time limit set by the device’s software) was measured and defined as “NIKBUT AVG”. Infrared meibography was performed in the upper and lower eyelids, and MGL was calculated using the meiboscore provided by the instrument that classifies the deficiency through a 0–3 scale: grade 0 = no gland loss; grade 1 = area of gland loss up to 33% of the total gland area; grade 2 = area of gland loss between 33% and 66%; and grade 3 = area of gland loss of 67% or more [24]. Ocular discomfort symptoms were scored by means of OSDI questionnaire.
Study Treatment
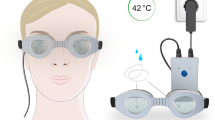
Patients were treated with four serial sessions performed every other day with a recently developed mask based on LLLT technology and dedicated for home use (my-mask®, Espansione Marketing S.p.A., Bologna, Italy). The instrument consists of two elements: (i) the device from which the treatments get set; (ii) the LM® LLLT terminal using red light (Fig. 1). A 3.5 mm enabler was removed when the device was handed to the patient in order to assign a maximum number of four sessions to avoid abuse (Fig. 2). Technical details concerning the device and the technology are reported in Table 1. During the study, patients received the first mask session in the office, while the remaining three sessions were performed at home during the evening. At T1, a counter of the mask sessions performed in the last week allowed one to check if patients used the device at home as recommended. The sequence of steps required for running the treatment is shown in Fig. 3. During the entire duration of the study, all patients were instructed to instill the same unpreserved hyaluronic acid 0.2%-based eye drops four times daily and to perform eyelid hygiene at bedtime. Concomitant use of other therapeutic agents for any ophthalmic disease was prohibited throughout the study period.
My-mask® is equipped with a 3.5 mm enabler to ensure that users do not abuse it beyond the recommended protocol of sessions. A If the enabler is inserted, my-mask® can be used without limits until exhausting the maximum number of treatments for the mask terminal (range 500–2000). B On the contrary, if the enabler is removed (e.g., when the device is handed to a patient), up to four treatments will be released. To run new treatments, the enabler must be inserted back into my-mask®
A To run a new treatment, my-mask® must be switched back on. B The mask terminal turns on—the treatment begins and the counter is kicked off. C After 17 min, treatment is completed, LM® LLLT mask turns off, the device’s screen stays on. D Turn off the my-mask® device through the smart remote or by disconnecting it from the power outlet
The logistic details of each diagnostic and therapeutic procedure conducted during the study are shown in Fig. 4.
Main Outcomes
The primary outcome measure was the improvement of OSDI score after the cycle of four mask sessions. Secondary outcome measure was the increase of NIKBUT (both first and average) at T1.
Sample Size
To determine the required sample size for the study, an a priori power analysis was performed that was based on the data of the study by Piyacomn et al. [25] Using this assumption, we calculated that a sample of 17 patients was required to detect a mean change of OSDI from baseline of 14.5 points, with a power of 0.80 and a P value of 0.05.
Statistical Analysis
SPSS statistical software version 22.0 (SPSS Inc, Chicago, Illinois) was used for data analysis. Values are expressed as mean ± standard deviation. The Shapiro–Wilk test and the Kolmogorov–Smirnov test were used to determine the normality of data. The Student’s t test was used to compare normally distributed continuous variables between T0 and T1, whereas the Wilcoxon signed-rank test was used for not normally distributed variables. A P value less than 0.05 was considered statistically significant.
Results
A total of 64 patients with DED were assessed for eligibility during the study period. Of these, 17 patients (3 male, 14 female; mean age 61.47 ± 11.93 years) fulfilled the criteria and were included in the study. All patients regularly completed the entire cycle of four mask sessions without the need for using prohibited medications. Therefore, data from all participants were included in the final analysis. Mean values of “NIKBUT first” and “NIKBUT average” increased from 5.29 ± 2.60 at T0 to 9.04 ± 3.49 s at T1 (P = 0.001) and from 9.40 ± 3.81 to 11.28 ± 2.81 s (P = 0.017), respectively; in parallel, the mean value of TMH increased significantly from 0.27 ± 0.06 to 0.32 ± 0.09 mm (P = 0.029). Conversely, there were no statistically significant differences for MGL (P = 0.346). Concerning ocular discomfort symptoms, OSDI score improved after treatment with a significant reduction of mean value from 32.00 ± 7.96 at T0 to 20.71 ± 8.03 at T1 (P < 0.001). No device-related adverse events were reported throughout the entire study.
Discussion
The present study reports the preliminary results of the first clinical experience with a novel portable device obtained by miniaturizing the in-office LLLT technology to provide patients with MGD with a small yet powerful solution to benefit from this therapy at home. After 1 week of treatment, NIKBUT (first and average) and TMH improved significantly; furthermore, thanks to the amelioration of ocular discomfort symptoms, as demonstrated by the significant improvement of OSDI score, patients were able to avoid the use of prohibited medications (e.g., corticosteroids) throughout the entire study.
The post-treatment improvements obtained with the novel mask used at home are consistent with those reported after the in-office use of the same technology (LLLT), both when used as a stand-alone therapy and in combination with IPL [17,18,19,20,21,22,23]. A recent prospective study that directly compared LLLT in conjunction to IPL versus tear substitutes demonstrated the superiority of device-assisted therapy over the topical one [26]. Clinical outcomes of LLLT are further supported by molecular changes detected in the tear fluid of patients who received the procedure. In fact, a significant reduction of interleukin-1β, interleukin-17F, and MMP9, MMP9/TIMP1 ratio, and ocular surface B cell proportions has been recently demonstrated [27].
It is not surprising that MGL was the only parameter that was not affected by the study treatment. In fact, it is very unlikely to obtain early changes in meibomian glands area after a short therapy [28], and more prolonged treatments are necessary to determine significant improvement of meibomian gland dropout [19].
This novel mask for home use adds some advantages to the current armamentarium of MGD therapies [29]. Firstly, it is independent with no need for other hardware/software. Secondly, it is a user-friendly “plug and play” device that does not require advanced know-how from operators or patients; thirdly, the procedure is painless and grants immediate relief to the patient. In order to implement the safety profile, the mask was equipped with an enabler to ensure that once the device is handed to the patient, the user cannot abuse it beyond the recommended protocol of four sessions.
In our opinion, the weekly protocol applied in this study could be used in the routine clinical practice in two main scenarios. Firstly, since treating with success MGD is often a matter of finding the right combination of in-office and at-home therapies, my-mask® could act as a boost to in-office procedures and/or conventional topical therapy. Secondly, iatrogenic DED represents a challenge, and my-mask® could be useful in patients undergoing ocular surgery (mainly cataract and refractive). In this setting, my-mask® could be used by the patient at home both before and after surgery in order to prevent the onset or mitigate the worsening of this complication.
The present study suffers from some limitations that deserve mentioning. The study design lacks a control group of patients treated with conventional topical therapy. However, we enrolled patients who were not satisfactorily controlled using first-line therapy (tear substitutes and eyelid hygiene). Thanks to the serial mask sessions, patients were able to better cope with ocular discomfort symptoms, as confirmed by the significant improvement of OSDI score, without requiring the use of other medications. Furthermore, since all patients were examined at baseline and after 1 week of treatment, the long-term effects of this treatment should be further investigated and more prolonged protocols of treatment should be explored.
Conclusions
A weekly protocol of serial sessions with a newly developed LLLT mask for home use allowed the significant improvement of the main objective parameters of the ocular surface (both tear production and stability) along with the significant amelioration of ocular discomfort symptoms. These results are consistent with those obtained with the in-office LLLT device and open up a new scenario for patients with MGD who can enjoy the unique benefits of this technology at home.
References
Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–83.
Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Investig Ophthalmol Vis Sci. 2011;52(4):1922–9.
Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology. 2017;124(11S):S20–6.
Gutgesell VJ, Stern GA, Hood CI. Histopathology of meibomian gland dysfunction. Am J Ophthalmol. 1982;94(3):383–7.
Liu S, Richards SM, Lo K, Hatton M, Fay A, Sullivan DA. Changes in gene expression in human meibomian gland dysfunction. Investig Ophthalmol Vis Sci. 2011;52(5):2727–40.
Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig Ophthalmol Vis Sci. 2011;52(4):1938–78.
Baudouin C, Aragona P, Messmer EM, et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf. 2013;11(4):246–58.
Bron AJ, Yokoi N, Gafney E, Tiffany JM. Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocul Surf. 2009;7(2):78–92.
Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for MGD. Investig Ophthalmol Vis Sci. 2011;52(4):1994–2005.
Greiner JV. A single LipiFlow® thermal pulsation system treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res. 2012;37:272–8.
Vigo L, Pellegrini M, Carones F, Scorcia V, Giannaccare G. Outcomes of serial sessions of Activa mask combined with intense pulsed light therapy in patients with Meibomian gland dysfunction. BMC Ophthalmol. 2022;22(1):313.
Vigo L, Pellegrini M, Carones F, Scorcia V, Giannaccare G. Short-term effects of a novel eye mask producing heat and vibration for the treatment of meibomian gland dysfunction: a pilot study. J Ophthalmol. 2021;2021:1370002.
Suwal A, Hao JL, Zhou DD, Liu XF, Suwal R, Lu CW. Use of intense pulsed light to mitigate meibomian gland dysfunction for dry eye disease. Int J Med Sci. 2020;17(10):1385–92.
Giannaccare G, Taroni L, Senni C, Scorcia V. Intense pulsed light therapy in the treatment of meibomian gland dysfunction: current perspectives. Clin Optom (Auckl). 2019;11:113–26.
Mittal R, Patel S, Galor A. Alternative therapies for dry eye disease. Curr Opin Ophthalmol. 2021;32(4):348–61.
Markoulli M, Chandramohan N, Papas EB. Photobiomodulation (low-level light therapy) and dry eye disease. Clin Exp Optom. 2021;104(5):561–6.
Di Marino M, Conigliaro P, Aiello F, et al. Combined low-level light therapy and intense pulsed light therapy for the treatment of dry eye in patients with Sjögren’s syndrome. J Ophthalmol. 2021;2021:2023246.
Pérez-Silguero MA, Pérez-Silguero D, Rivero-Santana A, Bernal-Blasco MI, Encinas-Pisa P. Combined intense pulsed light and low-level light therapy for the treatment of dry eye: a retrospective before-after study with one-year follow-up. Clin Ophthalmol. 2021;15:2133–40.
Park Y, Kim H, Kim S, Cho KJ. Effect of low-level light therapy in patients with dry eye: a prospective, randomized, observer-masked trial. Sci Rep. 2022;12(1):3575.
Solomos L, Bouthour W, Malclès A, Thumann G, Massa H. Meibomian gland dysfunction: intense pulsed light therapy in combination with low-level light therapy as rescue treatment. Medicina (Kaunas). 2021;57(6):619.
Stonecipher K, Abell TG, Chotiner B, Chotiner E, Potvin R. Combined low level light therapy and intense pulsed light therapy for the treatment of meibomian gland dysfunction. Clin Ophthalmol. 2019;13:993–9.
Marta A, Baptista PM, Heitor Marques J, et al. Intense pulsed plus low-level light therapy in meibomian gland dysfunction. Clin Ophthalmol. 2021;15:2803–11.
Giannaccare G, Pellegrini M, Carnovale Scalzo G, Borselli M, Ceravolo D, Scorcia V. Low-level light therapy versus intense pulsed light for the treatment of meibomian gland dysfunction: preliminary results from a prospective randomized comparative study. Cornea. 2022. https://doi.org/10.1097/ICO.000000000000299.
Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115(5):911–5. https://doi.org/10.1016/j.ophtha.2007.06.031.
Piyacomn Y, Kasetsuwan N, Reinprayoon U, Satitpitakul V, Tesapirat L. Efficacy and safety of intense pulsed light in patients with meibomian gland dysfunction—a randomized, double-masked, sham-controlled clinical trial. Cornea. 2020;39(3):325–32.
Meduri A, Oliverio GW, Tedesco G, Aragona P. Combined intense pulsed light and low-level light therapy for the treatment of refractory Meibomian gland dysfunction. Eur J Ophthalmol. 2022;13:11206721221127206.
D’Souza S, Padmanabhan Nair A, Iyappan G, et al. Clinical and molecular outcomes after combined intense pulsed light therapy with low-level light therapy in recalcitrant evaporative dry eye disease with meibomian gland dysfunction. Cornea. 2022;41(9):1080–7.
Dieckmann GM, Cox SM, Lopez MJ, et al. A single administration of OC-01 (varenicline solution) nasal spray induces short-term alterations in conjunctival goblet cells in patients with dry eye disease. Ophthalmol Ther. 2022;11(4):1551–61.
Borgia A, Raimondi R, Fossati G, et al. Device-based therapies as a boost of conventional treatment in dry eye disease. Expert Rev Ophthalmol. 2022. https://doi.org/10.1080/17469899.2022.2147928.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study. The rapid service fee was funded by the authors.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Sabrina Vaccaro, Marco Pellegrini, Massimiliano Borselli, Giovanna Carnovale Scalzo, Andrea Taloni, Rocco Pietropaolo and Ali Saeed Odadi. The first draft of the manuscript was written by Giuseppe Giannaccare and Adriano Carnevali. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Giuseppe Giannaccare, Sabrina Vaccaro, Marco Pellegrini, Massimiliano Borselli, Giovanna Carnovale Scalzo, Andrea Taloni, Rocco Pietropaolo, Ali Saeed Odadi and Adriano Carnevali confirm that they have no conflicts of interest to disclose.
Compliance with Ethics Guidelines
Informed consent was acquired from all the participants, and the study was carried out in accordance with the Declaration of Helsinki of 1964 and its later amendments, with approval from the local institutional ethics committee (Comitato etico Regione Calabria – Sezione Area Centro).
Data Availability
The data sets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Giannaccare, G., Vaccaro, S., Pellegrini, M. et al. Serial Sessions of a Novel Low-Level Light Therapy Device for Home Treatment of Dry Eye Disease. Ophthalmol Ther 12, 459–468 (2023). https://doi.org/10.1007/s40123-022-00619-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00619-3