Abstract
Introduction
A novel technique of using a sandwich-like structure, namely, an oral mucosa graft (OMG)–conjunctiva in situ–dermis-fat graft (DFG) (OMG-C-DFG), to reconstruct a contracted and low-capacity anophthalmic socket for a patient with ocular infection history was evaluated.
Methods
This was retrospective case study of four patients (cases) who underwent anophthalmic socket reconstruction surgery in which the OMG-C-DFG technique was applied. The procedures were performed in the Department of Ophthalmology at the Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine (Shanghai, China). Postoperative cosmetic appearance, graft outcome, the ability to wear an ocular prosthesis, and postoperative complications were evaluated.
Results
The median (± standard deviation) age of patients was 41.5 ± 22.1 (range 10–60) years. All patients suffered from contracted and low-capacity anophthalmic sockets. Three patients had a history of orbital implant infection and one patient had a history of enucleation due to exogenous endophthalmitis after globe rupture. The DFG and OMG were harvested from the abdominal region and lower lip, respectively. All four patients achieved a good postoperative appearance, with dermal surfaces appearing pink and smooth, the orbital areas showing good fullness, the ocular prosthesis showing good wearability, and no narrowing of the sockets. There was no lipid secretion, fat lysate outflow, or infection in the graft bed. There were only small amounts of scars and no infection of the donor site.
Conclusion
The sandwich-like structure can be effectively used to reconstruct the contracted and low-capacity anophthalmic socket with a history of orbital infection in one stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We present a novel surgical technique of using a sandwich-like oral mucosa graft (OMG)–conjunctiva in situ–dermis-fat graft (DFG) to reconstruct the anophthalmic socket with a history of ocular infection. |
This technique can solve both orbital volume deficiency and socket contraction in one stage, thereby reducing the time needed for surgery, shorten the treatment duration, decrease the financial burden of patients, accelerate the postoperative recovery, and improve patients’ satisfaction. |
Compared with artificial implants, DFG and OMG are both autologous tissues associated with better histocompatibility, better safety, lower risk of expulsion, and almost no risk of transmission of infectious diseases. |
Patients can wear ocular prostheses about 1 day after blepharotomy, which greatly enhances their appearance and benefits their mental and physical health. |
Introduction
Anophthalmic patients after enucleation not only have obvious functional deficits and facial deformities but also suffer from poor psychological outcomes [1]. Enucleation can result from complications, such as, for example, post-enucleation syndrome and phantom eye syndrome [2]. However, the psychological impact of this condition is widely underestimated [2]. Enucleation is usually undertaken with implant insertion, either as a primary or a secondary procedure. However, one of the most severe complications after implant insertion is implant exposure combined with infection [3]. As it is often difficult to notice the small region of exposure, by the time the patient comes to the clinic the exposure region has often become enlarged and infected. In cases of severe infection, treatment with systemic antibiotics can be inadequate, necessitating implant removal [4,5,6]. Orbital volume deficiency and socket contraction are common complications of implant removal. For patients with a history of orbital implant infection, the risk of infection increases if the implant is inserted again, and the duration of the operation may increase because of the need for multiple implant insertions after removal. There is the additional need to undergo yet another surgery to deal with the contracted conjunctival sac. Moreover, the implant is a variant component and is associated with the risk of rejection.
Surgeries that focus on solving orbital volume deficiency include secondary implants, subperiosteal implants, orbital fat grafting, dermis-fat grafting, and fillers, including calcium hydroxyapatite gel (Radiesse; Merz Pharma GmbH & Co. KGaA, Frankfurt, Germany), hyaluronic acid fillers, such as Restylane Sub-Q (Q-Med, Uppsala, Sweden) and Juvederm Voluma (Allergan, Irvine, CA, USA), and self-inflating pellet hydrogel expanders [7]. Treatments mainly focusing on solving socket contraction include socket moulders (specially designed, progressively larger conformers positioned with a firm pad), mucous membrane graft (MMG), amniotic membrane graft, auricular cartilage, hard palate graft, dermis-fat graft (DFG), vascularized pedicle flaps, fascia lata, and postauricular full thickness skin graft [3, 7]. However, reconstruction of a contracted and low-capacity anophthalmic socket can be a complex procedure, and one single procedure may not be sufficient for a good outcome [8, 9].
There have been several reports on surgical approaches for patients with a history of orbital infection, but to date there is no clear consensus on the preferred surgical technique and orbital reconstruction in these patients. Here, we report the novel technique of using a sandwich-like oral mucosa graft (OMG)–conjunctiva in situ–DFG (OMG-C-DFG) for the reconstruction of a contracted and low-capacity anophthalmic socket in patients for whom an orbital implant could not be placed or whose orbital implant was taken out due to infection, and evaluate its clinical effects. This kind of surgery can solve both orbital volume deficiency and socket contraction in one stage. In this report, we discuss the details of the surgical techniques and representative cases.
Methods
This is a retrospective case series. Four patients underwent anophthalmic socket reconstruction surgeries using the sandwich-like structure technique OMG-C-DFG. The surgeries were performed by the same two surgeons (Jin Li and Chunyi Shao) between May 2019 and December 2021, at the Department of Ophthalmology, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine (Shanghai, China). Inclusion criteria included patients with contracted and low-capacity anophthalmic sockets with a history of orbital infection, shortening of fornices, and inability to wear the prosthesis. Postoperative cosmetic appearance, graft outcome, ability to wear an ocular prosthesis, postoperative complications, and the need for additional surgery were evaluated. All patients had received definitive diagnoses and there was no change in interventions. This was a single-institution study which was approved by the Ethics Committee of Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine (ethics committee reference number: 2016-212-T161). This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study. Written informed consent was obtained from all participants before performing examinations and surgeries. All of the patients or their guardians gave specific consent for the publication of their data and images. The CARE reporting guidelines were followed [10].
Surgical Technique
Preparation for Surgery
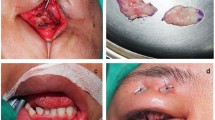
With the patient under general anesthesia, two 3-0 silk sutures are separately passed through the gray lines of the upper and lower lid margins to provide traction (Fig. 1a). The contracted conjunctival socket is then incised horizontally and dissected to make a cavity to ensure that the socket is sufficient wide. The conjunctiva is not detached from the deep tissues. The cavity volume is then calculated for preparation of DFG (Fig. 1b).
Photos of the surgical procedure. a The contracted and low-capacity anophthalmic socket. b The cavity provided for the dermis-fat graft (DFG) implant. c Removal of the epidermal tissue of the donor area by using the grinding head of the power system. d Complete removal of the dermis. e Placement of the DFG into the orbital cavity. f Interrupted suturing of the dermis and the conjunctiva in situ around the cavity. g Use of methylene blue to mark the donor area of the lower lip. h Complete removal of the oral mucosa. i Cover and interrupted suturing of the oral mucosa graft (OMG) donor site with Heal-full. j Placement of the OMG into the conjunctiva-deficient area. k Use of the conformer to expand the OMG. l Use of two foams as pads to prevent the skin from being cut after tarsorrhaphy
Harvesting and Implantation of DFG
The DFG is harvested from the abdominal skin on the lateral and inferior of the navel. An ellipse of tissue to be harvested is marked (approx. 25 × 20 mm for adults and a bit smaller for children); the surface area of the dermis graft should be 20% larger than the surface area of the cavity. The grinding head of the power system is used to remove the epidermal tissue of the donor area (Fig. 1c). A sharp knife must be used to remove a thin layer of the dermis; hair follicles are then removed and the skin incised along with the markings (Fig. 1d). Scissors are used to dissect a plug of dermis and fat, approximately 20 mm in depth. The donor site are closed in layers. The donor area is sutured subcutaneously with 5-0 absorbable suture, and the skin is sutured with 5-0 silk suture. The DFG is placed into the cavity that has been created and then trimmed to achieve the appropriate size and fat content (Fig. 1e). 8-0 absorbable suture is used to connect the dermis and the conjunctiva around the cavity by interrupted suturing (Fig. 1f).
Harvesting and Implantation of OMG
The conjunctiva is incised along the upper and lower fornices, following which the tissues are separated vertically downwards. A transparent and thin conformer, which is larger than the standard lamel, is placed in the conjunctival sac so that the eyelid margin will be able to close entirely. Finally, the defective area of the conjunctiva is measured. Methylene blue is used to mark the donor area of the lower lip (Fig. 1g). The donor area should be a bit larger than the conjunctiva defective area. The full-thickness of OMG is then harvested from the lower lip; this procedure should be carried out with caution to avoid the labial glands (Fig. 1h). The harvested graft is trimmed at the margin and the submucosal tissue is removed with scissors. The donor area is covered by Heal-full (an acellular bovine dermal matrix; ZH-BIO, Yantai, China) and sutured with a 5-0 absorbable suture or a 5-0 silk suture (Fig. 1i). The size of the OMG is then modified to that of the defect and sutured interruptedly to the surrounding conjunctiva with an 8-0 absorbable suture (Fig. 1j). A transparent and thin conformer is then placed in the conjunctival sac to make OMG expand entirely (Fig. 1k).
Tarsorrhaphy
The middle half of the epithelial layer of the upper and lower eyelid margins is scrapped off, and they are split along the gray line. The posterior layer of the eyelid margin is sutured with a 5-0 absorbable suture. The anterior layer of the eyelid margin is sutured with 3-0 silk thread mattress sutures, and two foams of 7-0 thread plate are used as thread pads to prevent the skin from being cut (Fig. 1l). Tobramycin- and dexamethasone-containing eye ointment is squeezed into the conjunctival sac and applied to the eyelids. A gauze is placed over the area with gentle pressure.
Postoperative Management
Levofloxacin eye drops are used four times a day for 2 weeks, and tobramycin- and dexamethasone-containing eye ointment is applied every night for 1 week. Preservative-free artificial tears are used three times a day for 4 months. The wounds are cleaned every day. The pressure patch is kept in place for 7 days and changed every day. The 3-0 silk threads are removed from the anterior layer of the eyelid margin after 2 weeks, and the sutures from the DFG and OMG donor sites are also removed after 2 weeks. Blepharotomy is performed 4 months later under local anesthesia using an electric knife to stop bleeding if necessary. The patient can wear an ocular prosthesis on the second day after blepharotomy.
Results
Four patients with contracted and low-capacity anophthalmic sockets underwent reconstructive surgery using the sandwich-like structure technique (Fig. 2) OMG-C-DFG. All four patients had undergone enucleation and had a history of ocular infection. Three patients had implanted orbital implants and had taken them out due to implant infection and exposure. One patient had a history of exogenous endophthalmitis after globe rupture. The median (± standard deviation) age of patients was 41.5 ± 22.1 (range 10–60) years (Table 1). The follow-up period ranged from 23 to 32 months. Three patients had anophthalmic sockets in the right eye, and one patient had an anophthalmic socket in the left eye. The DFGs were harvested from the abdominal region, and the OMGs were harvested from the lower lip.
All four patients achieved live DFGs and OMGs with reliable blood supply and without graft atrophy and contraction. The dermal surfaces appeared pink and smooth and were covered by migrated conjunctival epithelium without dermis ulceration, hirsutism, keratinization, and lipid secretion. The fat provided sufficient volume with good fullness and without fat hypertrophy, atrophy, lysate outflow, and infection. The OMG grew well and smooth with enough surface area and good histocompatibility of the conjunctivae in situ. The conjunctivae in situ grew well with good histocompatibility of both DFG and OMG and without atrophy and contraction. The patient needs to be carefully observed for potential postoperative complications, such as graft atrophy, infection, ulceration, fat lysate outflow, ptosis, recurrent socket contraction, graft hirsutism, keratinization, and conjunctival cysts or granulomas. None of these complications were observed in these four patients [11].
All four patients achieved good postoperative appearances with good fullness in the orbital areas, good wearability of the ocular prosthesis, and good symmetry of their eyes (Figs. 3, 4). They all expressed a high degree of satisfaction with their appearance after the procedure (Table 1) [12], which greatly contributed to a rebuilding of confidence and was beneficial to their physical and mental health.
Photos of case 1. a Preoperative photo with contracted and low-capacity anophthalmic socket. b Preoperative photo of anterior segment with loss of fornices. c Postoperative photo after OMG-C-DFG placement and prosthesis fitting. d Postoperative photo with glass wearing. e Postoperative photo with enough space for the upper and lower fornices
Discussion
Multiple methods are available for the reconstruction of anophthalmic sockets. However, reconstruction and rehabilitation of a contracted and low-capacity anophthalmic socket often represent a challenge to even the most experienced surgeon and ocularist [8]. Few reports have been published on patients with orbital implant infection or a history of exogenous endophthalmitis. Chiu et al. reported that the failure rate of orbital evisceration with primary implant placement in acutely infected/inflamed eyes in their study population was 7.8% (SD 8.0%, 95% confidence interval 2.7–12.9%, range 0–27%) [13]. The treatment for a common anophthalmic socket is orbital implant placement, but this treatment has a number of limitations in cases of patients with a history of ocular infection. These limitations include: (1) risk of rejection and exposure due to the orbital implants consisting of alloplastic materials; (2) in patients with a history of ocular infection, the risk of reinfection with re-implanation of an orbital implant; (3) the need to first deal with volume enlargement and then a second surgery to deal with the shortened fornix, i.e. prolongation of treatment duration.
According to previous research, DFG has multiple indications, such as congenital anophthalmia [14, 15], contracted anophthalmic socket (if there is enough vascularized tissue) [16, 17], enucleation per primam or per secundam [13], chronic anophthalmic socket pain [7, 18], as primary and secondary orbital implants for both adults [19] and children [20], anophthalmic socket in children enucleated for RB [21], early implant exposure [22], superior sulcus reconstruction [23, 24], fornix reconstruction [25], and eyelid malposition [15]. Smith et al. proposed that the advantage of the DFG lies in its ability to replace the orbital volume, while, at the same time, maintaining the fornix and conjunctiva [26]. Therefore, DFG can be used to treat not only orbital volume deficiency but also socket contraction.
However, Aryasit et al. analyzed six adult patients who underwent primary DFGs with concurrent infection; they noted a 50% failure rate due the severity of the conjunctival defect and a high risk of further socket retraction [27]. Aryasit et al. also analyzed 35 patients under secondary DFGs, of whom nine patients underwent fornix reconstructions by using the mucous membrane or hard-palate graft. However, the combined procedure was successful in only 25% of cases because of insufficient blood supply from the base leading to mucous–membrane graft shrinkage. As a result, the surgeon opted for a DFG with an extended dermis to replace the superior and inferior fornix [27]. In a recent study, Hayat et al. evaluated the outcomes of secondary autologous DFG as an orbital implant in anophthalmic sockets; of the 12 patients, two went into failure because of a history of radiotherapy, with fornix contraction with fat resorption after DFG, and developed fornix scarring conformer extrusion. One was additionally treated by buccal mucosal graft and redo DFG, and the other by redo DFG and amniotic membrane graft [28]. Lin et al. reported the results of autogenous DFG for the management of extremely large-area implant exposure. In their study, 30 eyes with large-area exposure were managed with DFGs; of these, 80% were successfully treated with a single surgery and 20% developed fornix loss and required additional reconstruction with a full-thickness skin graft [3].
These studies demonstrate that reconstruction of the contracted and low-capacity anophthalmic socket can be a complex procedure, and that one single procedure may not suffice [8, 9]. Other procedures have been reported, such as the composite hard palate and DFG together with adjunctive use of 5-fluorouracil injections [8], porous orbital implant with MMG [16], dental molding compound wrapped with split-thickness skin or mucosal graft [29], and a meshed skin graft in conjunction with a semi-rigid conformer-stent, orbital osteotomy, and free flap transfer [30].
Several surgical techniques have been reported, but to date no clear consensus exists on the preferred surgical technique and orbital reconstruction for patients with a history of ocular infection history. Here, we report and describe a novel technique involving the use of a sandwich-like structure, namely, the OMG-C-DFG. The good blood supply of the OMG-C-DFG supports survival of the OMG and DFG. It is possible that if we suture the DFG and OMG together, the survival rate of both might decrease. Before we perform tarsorrhaphy, we place a transparent and thin conformer into the orbit for support for 4 months. The contraction of scars usually happens within 1–3 months following surgery. Using our sandwich-like structure, we observed no contraction during the 3 months post-surgery and the DFG and OMG may not contract even after that period; thus, the odds of socket contraction recurrence are decreased. The three parts of the sandwich-like structure have different functions. The fat provides enough replacement which can restore the orbital volume and relieve enophthalmos; the dermis integrates into the recipient environment to facilitate vascularization, providing vascular support for the graft, to provide rigidity for suturing and a matrix for mucosal epithelialization [13]; and the OMG plays a role in increasing the surface area and depth of the fornix. The OMG is a fresh transplantation of autologous tissue, with intact mucosal epithelium and abundant vascularity, which makes the grafts easy to attach and facilitates survival. The OMG can directly replace the conjunctival tissue to form a conjunctival sac. At the same time, the conjunctivae in situ are protected and utilized to the maximum extent so that they can fully extend to increase the orbital surface areas.
There are multiple advantages to using this sandwich-like structure. First, compared with artificial implants, DFG and OMG are both autologous tissues with better histocompatibility, better safety, lower risk of expulsion, and almost no risk of transmission of infectious diseases. Second, the risk of infection will decrease. Third, this technique can solve both orbital volume deficiency and socket contraction in one stage, thereby reducing the time needed for surgery, shortening the treatment duration, decreasing the financial burden of patients, accelerating the postoperative recovery and improving patients’ satisfaction. Finally, patients can wear ocular prostheses about 1 day after blepharotomy, thus enhancing their appearance and improving their mental and physical health.
However, there are a number of disadvantages to this procedure. Harvesting of both OMG and DFG requires separate surgical sites with associated donor site morbidity. The disadvantages of the donor site include a second site being added to the surgical wound, source of postoperative pain in the early period, and the formation of postoperative scars in the late period. Regarding the OMG donor site, the disadvantages include prolonged oral bleeding and postoperative pain. We have used Heal-full instead of directly suturing the wounds together to reduce the pain and accelerate wound healing. The labial mucosa is easier to obtain than the buccal mucosa. The former has a larger surface area and thinner mucous membrane than the buccal mucosa. In addition, the surface of the labial mucosa is smooth, and it is more convenient when Heal-full is used to cover and protect the wounds, relieve the pain and accelerate the healing of labial mucosa. Heal-full can dissolve and fall off within about 3 weeks. The wounds of the lower lip will be covered by migrated oral mucosa epithelium, with only small scars of the wounds remaining; thus there is no interference with the lip shape and the ability to eat. However, these limitations remain rare, and the procedures are generally well tolerated with minimal long-term morbidity.
We suggest marking the ellipse of tissue to be harvested (approx. 25 × 20 mm for adults and smaller for children). The surface area of the dermis graft should be 20% larger than the surface area of the cavity. The volume of the DFG should be calculated carefully. Selection of the donor size is also necessary. If the DFG were to be too small, it might lead to under-correction and enophthalmos would not be successfully treated. If the DFG were to be too large, fat lysate outflow, expulsion, and central necrosis may occur due to compression and ischemia. We harvest the DFG from the abdominal area, and the hairs and sebaceous glands should be removed to avoid hair growing and lipid secretion in the graft area. It is also necessary to check if the patient has enough fat panicle and has no skin infections. Careful site selection and layered closure help to prevent DFG donor site dehiscence. The OMG is harvested from the lower lips, and we suggest using Heal-full to accelerate the healing of oral mucosae and protect the wounds and release the pain. We also suggest putting a transparent and thin conformer into the orbit for support for 4 months to avoid socket contraction, as well as covering and pressing the wounds with gauze to prevent hematomas.
The limitations of our study include the small sample size and the short follow-up periods. Although our four patients all achieved satisfactory outcomes, we still need to follow them for possible complications in the long term.
Conclusions
The novel sandwich-like structure described here, the OMG-C-DFG, can be effectively used in the one-stage reconstruction of a contracted and low-capacity anophthalmic socket with orbital infection history by restoring volume and expanding the fornix to allow successful prosthesis retention both in adults and children. We believe the sandwich-like structure is an excellent and effective option for anophthalmic socket reconstruction, particularly in cases with a history of ocular infection and contracted and low-capacity sockets.
References
Wang KJ, Li SS, Wang HY. Psychological symptoms in anophthalmic patients wearing ocular prosthesis and related factors. Medicine (Baltimore). 2020;99(29):e21338.
Martel A, Baillif S, Thomas P et al. Phantom vision after eye removal: prevalence, features and related risk factors. Br J Ophthalmol. 2021.
Lin CW, Liao SL. Long-term complications of different porous orbital implants: a 21-year review. Br J Ophthalmol. 2017;101(5):681–5.
Jordan DR, Brownstein S, Jolly SS. Abscessed hydroxyapatite orbital implants. Ophthalmology. 1996;103(11):1784–7.
Soparkar CNS, Patrinely JR. Abscessed hydroxyapatite orbital implants. Ophthalmology. 1997;104(7):1784–7.
Fu L, Patel BC. Enucleation. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK562144/.
Quaranta-Leoni FM, Fiorino MG, Quaranta-Leoni F, Di Marino M. Anophthalmic socket syndrome: prevalence impact and management strategies. Clin Ophthalmol. 2021;15:3267–81.
Choi CJ, Tran AQ, Tse DT. Hard palate-dermis fat composite graft for reconstruction of contracted anophthalmic socket. Orbit. 2019;38(3):199–204.
Chiu SJ, Tan JHY, Currie ZI. To implant or not to implant: emergency orbital eviscerations with primary orbital implants. Eye (Lond). 2021;35(11):3077–86.
Gagnier JJ, Kienle G, Altman DG et al. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013;2013:bcr2013201554.
Starks V, Freitag SK. Postoperative complications of dermis-fat autografts in the anophthalmic socket. Semin Ophthalmol. 2018;33(1):112–5.
Klassen AF, Cano SJ, Scott A, Snell L, Pusic AL. Measuring patient-reported outcomes in facial aesthetic patients: development of the FACE-Q. Facial Plast Surg. 2010;26(4):303–9.
Schmitzer S, Simionescu C, Alexandrescu C, Burcea M. The anophthalmic socket—reconstruction options. J Med Life. 2014;7 Spec No.4:23–9.
Modugno AC, Resti AG, Mazzone G, et al. Long-term outcomes after cosmetic customized prostheses and dermis fat graft in congenital anophthalmia: a retrospective multicentre study. Eye (Lond). 2018;32(12):1803–10.
Jovanovic N, Carniciu AL, Russell WW, Jarocki A, Kahana A. Reconstruction of the orbit and anophthalmic socket using the dermis fat graft: a major review. Ophthalmic Plast Reconstr Surg. 2020;36(6):529–39.
Bhattacharjee K, Bhattacharjee H, Kuri G, Das JK, Dey D. Comparative analysis of use of porous orbital implant with mucus membrane graft and dermis fat graft as a primary procedure in reconstruction of severely contracted socket. Indian J Ophthalmol. 2014;62(2):145–53.
Peseyie R, Raut AA. Contracted Socket. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK568764/.
Shams PN, Bohman E, Baker MS, Maltry AC, Kopp ED, Allen RC. Chronic anophthalmic socket pain treated by implant removal and dermis fat graft. Br J Ophthalmol. 2015;99(12):1692–6.
Baum SH, Schmeling C, Pfortner R, Mohr C. Autologous dermis—fat grafts as primary and secondary orbital transplants before rehabilitation with artificial eyes. J Craniomaxillofac Surg. 2018;46(1):90–7.
Quaranta-Leoni FM, Sposato S, Raglione P, Mastromarino A. Dermis-fat graft in children as primary and secondary orbital implant. Ophthalmic Plast Reconstr Surg. 2016;32(3):214–9.
Bosch-Canto V, Cruz C, Ordaz-Favila JC. Dermal-fat graft for anophthalmic socket in children enucleated for retinoblastoma. Archiv Soc Esp Oftalmol (English Edn). 2018;93(1):3–6.
Lu YL, Chen ZT, Tsai IL. Dermis-fat graft as treatment of early implant exposure in a postpenetrating keratoplasty patient with nontraumatic eyeball rupture. Taiwan J Ophthalmol. 2020;10(2):134–7.
Van Gemert JV, Leone CR. Correction of a deep superior sulcus with dermis-fat implantation. Arch Ophthalmol. 1986;104(4):604–7.
Czyz CN, Foster JA, Wulc AE. Superior sulcus volumetric rejuvenation utilizing dermis fat grafting. Aesthet Surg J. 2015;35(7):892–8.
Lopes N, Castela G, Andres R, Lisboa M, Castela R, Loureiro R. Reconstruction of anophthalmic socket. J Fr Ophtalmol. 2011;34(9):608–14.
Smith B, Bosniak S, Nesi F, Lisman R. Dermis-fat orbital implantation: 118 cases. Ophthalmic Surg. 1983;14(11):941–3.
Aryasit O, Preechawai P. Indications and results in anophthalmic socket reconstruction using dermis-fat graft. Clin Ophthalmol (Auckland, NZ). 2015;9:795–9.
Hayat N, Jan S, Atiq N, Cheema A. Outcomes of secondary autologus dermo-fat orbital implants in anophthalmic sockets. Pak J Med Sci. 2021;37(2):426–31.
Mavrikakis I, Malhotra R, Shelley MJ, Sneddon KJ. Surgical management of the severely contracted socket following reconstruction. Orbit. 2006;25(3):215–9.
Zhang R. Reconstruction of the anophthalmic orbit by orbital osteotomy and free flap transfer. J Plast Reconstr Aesthet Surg. 2007;60(3):232–40.
Acknowledgements
Funding
Sponsorship for this study and the journal’s Rapid Service Fee was funded by the National Nature Science Foundation of China (81870688, 81970834, and 81500765), Shanghai Jiao Tong University School of Medicine Two-hundred Talent (20191914), and the Funding of Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine (JYLJ202008).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by QQ, RL, CS, and JL. The first draft of the manuscript was written by QQ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. QQ and RL contributed equally to this paper and should be considered as co-first authors.
Disclosures
Qiaoran Qi, Rui Li, Yue Wu, Yu Yu, Ming Lin, Chunyi Shao, and Jin Li declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine (ethics committee reference number: 2016-212-T161). This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study. Written informed consent was obtained from all participants before performing examinations and surgeries. All of the patients or their guardians gave specific consent for the publication of their data and images.
Data Availability
All data generated or analyzed during this study are included in this published article.
Thanking Patient Participants
We thank every study participant for their involvement in the study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Qi, Q., Li, R., Wu, Y. et al. A Sandwich-Like Oral Mucosa Graft–Conjunctiva In Situ–Dermis-Fat Graft for Reconstruction of the Anophthalmic Socket with Ocular Infection History. Ophthalmol Ther 11, 1261–1271 (2022). https://doi.org/10.1007/s40123-022-00500-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00500-3