Abstract
Introduction
To compare the influence of one microkeratome and three femtosecond lasers on myopic laser in situ keratomileusis (LASIK) outcomes.
Methods
Retrospective, observational cohort study. We compared 134 eyes treated with the IntraLase 60 kHz, 112 eyes treated with the Femto LDV Z6, 206 eyes treated with the FS200, and 98 eyes treated with the Hansatome zero compression microkeratome. All eyes were operated on using the same surgical protocol with the same excimer laser (Wavelight Allegretto) and were allocated to refraction-matched groups.
Results
One day and one week postoperatively, uncorrected distance visual acuity was significantly lower in the FS200 group compared to others (P = 0.0001). This difference disappeared at the 1- and 3-month postoperative visits. Significant differences were found among groups in terms of safety index (P = 0.0001), residual sphere (P = 0.0001), and residual cylinder (P = 0.02) at the 3-month postoperative visit. No significant differences were found in corrected distance visual acuity or efficacy index.
Conclusion
According to our results, a slight delay in visual restoration after FS200 LASIK surgery might be expected. This delay was statistically significant at 1 day and 1 week postoperatively, but there were no differences from the 1-month visit onwards. Additionally, significant differences were found among devices in terms of safety index and the refractive results, which were found not to be clinically relevant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Why carry out this study? |
Different femtosecond laser platforms are now available to perform femtosecond laser-assisted in situ keratomileusis (FS-LASIK) as an alternative to mechanical microkeratome. The corneal flap created by each device seems to have different characteristics that may result in different visual and refractive outcomes, speed of recovery, and visual quality. |
Given the scarce published literature comparing more than two devices to perform myopic LASIK, we decided to design a study in a young population (less than 40 years of age), performed by two experienced surgeons, and using three different femtosecond platforms, one mechanical microkeratome, and the same excimer laser for all groups. |
What was learned from the study? |
Both IntraLase®, Femto LDV®, FS200® femtosecond lasers, and Hansatome® mechanical microkeratome are safe and effective for flap creation in laser refractive surgery for myopia when combined with the Allegretto® excimer laser, although slight differences in visual and refractive outcomes are found at the 3-month postoperative visit. In addition, a slower visual acuity recovery is found in FS200-treated eyes. |
On the basis of the results, we found statistically significant differences in terms of residual refraction and safety among these devices, and additionally the speed of visual recovery might also be different. |
Introduction
Laser in situ keratomileusis (LASIK) is the gold standard among refractive surgery techniques for the surgical correction of myopia [1, 2]. With the advent of femtosecond lasers and their wide adoption in clinical practice, the use of mechanical microkeratomes (MM) has declined in recent years. As a consequence, MM-related flap complications have become less frequent [3, 4]. Compared to flaps cut with MM, flaps created with femtosecond lasers are more predictable in terms of attempted thickness and homogeneity [2, 5], are associated with fewer higher-order aberrations [6,7,8,9], offer higher contrast sensitivity [7, 8], induce less dry eye [10], and afford higher corneal biomechanical stability [11]. Owing to these important clinical advantages, nowadays 70% of LASIK procedures are performed using a femtosecond laser [1].
Most of the published studies on femtosecond LASIK performance were conducted with the IntraLase® laser (Abbott Medical Optics Inc., Santa Ana, California), as this was the first and only available device for some years. Although its efficacy, safety and predictability have been repeatedly demonstrated [4, 5, 12], the available published evidence for some of the more recently developed femtosecond platforms remains limited. A paper by the American Academy of Ophthalmology [5] reviewing the use of femtosecond laser (IntraLase®) versus MM concluded that outcomes with the former were as good as or better than with the latter for flap creation, and encouraged more studies in order to compare efficacy outcomes of newer femtosecond platforms by other manufacturers. Clinically relevant differences in outcomes might be expected among femtosecond systems because of variations in photodisruption characteristics, flap morphology, energy transmission, gas management, etc. Overall, these technical differences could induce dissimilar tissue responses specific to each femtosecond laser.
Most of the published studies comparing different femtosecond platforms have been designed to compare flap morphology and predictability or intraocular pressure elevations during the procedure [13,14,15]. Unfortunately, there is less published evidence on visual and refractive results with these newer femtosecond lasers. This is clinically important, as satisfactory clinical outcomes should not be taken for granted, especially if new laser platforms have not been adequately compared against existing, well-established options. A recently published review and meta-analysis [16] showed some significant differences among femtosecond platforms in terms of efficacy, predictability, and flap complications. In previous studies, certain methodological issues that could have influenced the outcomes should be taken into account (e.g. the different excimer laser platforms that were used for stromal ablation).
In order to control these potential biases, we designed a specific study protocol that basically consists in using the same surgical protocol, the same excimer laser, and to include refraction matched eyes.
Methods
This was a retrospective cohort study of patients younger than 40 years who underwent LASIK with MM or a femtosecond laser for the correction of myopia with or without astigmatism between 2008 and 2014.
A masked investigator performed the preoperative examination that included uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA) (Nidek autochart projector CP 670, Nidek, Gamagori, Japan), manifest and cycloplegic refraction, ultrasound corneal pachymetry (DGH 5100 contact pachymeter, DHG Technology Inc, Exton, PA; OcuScan RXP, Alcon Laboratories, Inc, Fort Worth, TX), topography/tomography and keratometry (Dicon CT200, Vismed Inc., San Diego, CA; CSO Construzione Strumenti Oftalmici, Italy), mesopic infrared pupilometry (Colvard Pupillometer, Oasis 78 Medical Inc., Glendora, CA), slit-lamp biomicroscopy, Goldmann tonometry, and dilated funduscopy.
Exclusion criteria were unstable refraction, pachymetry under safety limits or suspicion of keratoconus or other ectatic corneal condition (defined as any localized steepening documented with Placido corneal topography or bowing of the posterior corneal surface detected with corneal tomography), prior ocular surgery, or systemic diseases that could alter refractive or visual outcomes.
The choice of a femtosecond or the MM depended mainly on the preoperative keratometric measures and the pupil size. For keratometric measurements less than 41.0 diopters (D) or greater than 46.0 D and pupil diameter at least 7 mm, the flap was always created with a femtosecond laser. In patients suitable for both procedures, the final decision was based on the patient’s preference after being thoroughly informed about both techniques. The patients were allocated to one of the three femtosecond groups depending on device availability in the facilities at the time of surgery.
All patients provided informed consent and the institutional review board approved the study protocol (regional committee of clinical research of the Community of Madrid. REF 216/3). The study was performed in accordance with the tenets of the Declaration of Helsinki.
Surgical Technique
Two experienced surgeons (M.A.T. and M.G.G.) performed all the procedures in a private practice setting.
Povidone-iodine solution 5% was applied on the eyelids and conjunctiva before the sterile surgical drape and eyelid rigid speculum were positioned. All surgeries were performed under topical anaesthesia (lidocaine 2%).
In eyes treated with the MM (group H), the flap was cut with the Hansatome Zero-compression® keratome (Hansa Research and Development, Miami, FL, USA and commercialized by Bausch and Lomb Corporation) using a 8.5–9.5 mm suction ring, a 120-μm blade, and superior hinge.
In the femtosecond groups three platforms were used: (a) the 60-kHz IntraLase® laser (group IL), programed for raster pattern photon delivery, bed energy level of 0.90 μJ, side-cut energy of 0.90 μJ, spot separation of 7 μm, side cut angle of 70°, superior hinge angle of 50°, attempted flap depth of 110 μm, and flap diameter of 8.5 mm; (b) the Wavelight FS200® laser (group F) by Alcon Laboratories, Inc. Fort Worth, TX, USA, programed for raster pattern photon delivery, bed energy level of 0.83 μJ, side-cut energy of 0.80 μJ, spot separation of 8 μm, side cut angle of 70°, superior hinge angle of 90°, attempted flap depth of 120 μm, and flap diameter of 9.0 mm; (c) the Femto LDV Z6® (group Z) by Ziemer Ophthalmic Systems AG, Port, Switzerland, programed for raster pattern photon delivery, bed energy level of 1.0 μJ, side-cut energy of 0.90 μJ, spot size of 1 μm, side cut angle of 70°, superior hinge angle of 90°, attempted flap depth of 110 μm, and flap diameter of 9.0 mm. A suction ring of 9–10 mm was used depending on the corneal curvature according to the manufacturer’s recommendations.
In all groups, once the flap was cut, it was lifted with a spatula and the stromal bed was dried with a sponge. The stromal ablation was performed with the Wavelight Allegretto® excimer laser (WaveLight Laser Technologies AG) programed for spot separation of 0.95 mm, fluence of 200 mJ/cm2, repetition rate of 400 Hz, optical zone of 6–7.5 mm (larger than or equal to the patient’s mesopic pupillary size), and conventional treatment (non-customized) according to the manufacturer’s recommendations.
After the ablation, the residual stromal bed was gently rinsed with balanced salt solution (BSS®, Alcon Laboratories Inc., Ft. Worth, TX) and the flap was repositioned over the stromal bed. Antibiotic drops (ciprofloxacin 3 mg/mL, Oftacilox®, Alcon Cusí, Barcelona, Spain) and non-steroidal anti-inflammatory eyedrops (ketorolac trometamol 5 mg/mL, Acular®, Allergan, Madrid, Spain) were instilled before the speculum was removed.
Postoperative Follow-Up
Ciprofloxacin 3 mg/mL and steroid drops (dexamethasone alcohol 1 mg/mL, Maxidex®, Alcon Cusí, Barcelona, Spain) were prescribed four times daily during the first postoperative week and preservative-free artificial tears were applied as needed.
All patients were examined at 1 day, 1 week, and 1 and 3 months postoperatively by two experienced masked optometrists who recorded UDVA and CDVA in the same room using the same light adjusted to mesopic conditions. At the 3-month visit, a complete ocular examination was performed, including manifest residual refraction, CDVA, and topography.
Statistical Analysis
Statistical analysis was performed with the “Statview SE + Graphics” program (Abacus Concepts Inc., Berkeley, CA, USA) for Macintosh.
Visual acuity was measured on the decimal scale (Snellen values) but converted to logMAR for statistical analysis using a conversion chart.
The Kolmogorov–Smirnov test was used to test normality and factorial analysis of variance (ANOVA) was used for multiple comparisons analysis. Intra-group linear regression analysis was performed. The 95% confidence intervals (CIs) were set up and P values less than 0.05 were considered statistically significant.
Results
A total of 550 myopic eyes were included and were allocated to one of four refraction-matched groups: 134 eyes were allocated to group IL, 112 eyes to group Z, 206 eyes to group F, and 98 eyes to group H. The preoperative sphere and cylinder were matched within ± 0.50 diopters (D) between groups.
Preoperative data are shown in Table 1; preoperative sphere range was − 0.75 to − 7.75 D, and cylinder was no greater than − 4.5 D. Some statistically significant differences were found in terms of CDVA, keratometry, and age, due to a large sample size of the study. Nevertheless, CDVA was at least 1.0 (decimal) in all patients preoperatively, and the mean age of the sample was younger than 40 years; therefore, these differences were considered not to be clinically relevant.
Statistically significant differences in UDVA (both in decimal and logMAR notations) were noted among the groups in the 1-day and 1-week postoperative visits (eyes in group F had lower UDVA than all other groups, P = 0.001), but these differences were not significant at the 1-month and 3-month postoperative visits (Table 2). Similarly, no statistically significant differences in CDVA were found among groups at the 3-month postoperative visit (Table 3).
The myopic residual sphere in eyes of group F was significantly higher (P = 0.0001) compared to eyes of the rest of the groups (Table 3). The residual cylinder in eyes of group F reached emmetropia, while pairwise comparisons revealed that there was statistically significant difference in residual cylinder only for the comparison between eyes of group IL and group F (P = 0.02, Table 3).
Figure 1 depicts UDVA data 3 months after surgery. The mean change in lines between preoperative and postoperative CDVA for the groups is shown in Table 3.
The efficacy index was similar in all groups, but a tendency for significance was detected between eyes in group F versus those in group IL and versus those in group H (Table 3). Regarding the safety index, statistically significant differences were found between eyes in group F versus those in group IL and group H (P = 0.0001). No statistically significant differences in the safety index were detected between eyes in groups F and Z (Table 3). None of the patients lost more than two lines of CDVA. Other changes in lines of CDVA are summarized in Fig. 2.
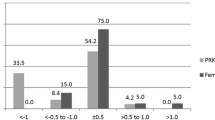
The predictability of the residual spherical equivalent (SE) within 1.0 D and within 0.5 D was similar (P = 0.04 and P = 0.5, respectively) among the groups (Fig. 3).
Linear regression analysis showed a positive, statistically significant relationship between preoperative SE and the effectively corrected refraction in all groups (Fig. 4).
Attempted versus achieved spherical equivalent refraction scatterplots 3 months after LASIK for myopia correction for the IntraLase (a), Femto LDV (b), FS200 (c), and Hansatome (d) groups. The linear regression equation and coefficient of determination (R2) are displayed. Intra-group linear regression analysis test. LASIK laser in situ keratomileusis, D diopters
Discussion
We found a slight delay in visual recovery after myopic LASIK in eyes of group F in the early follow-up visits. This delay was statistically significant at 1 day and 1 week postoperatively, but there were no differences from the 1-month visit onwards.
In all groups, visual acuity improved throughout the follow-up visits. Eyes in group Z achieved better UDVA with minimal standard deviation in all visits, except the third month visit where it was surpassed by eyes in group F. In accordance with this finding, a slightly higher efficacy index was noted for eyes in group F.
Several studies have been published comparing the use of femtosecond lasers versus MM [1, 2, 4, 6, 7, 13, 17,18,19,20]. The existing evidence suggests that femtosecond lasers are at least comparable to MM [5], or even superior in terms of predictability [2], visual restoration [13], flap morphology [5], higher-order aberrations [1], and intraoperative safety profile [2, 5].
Refractive and visual outcomes after IntraLase LASIK have been reported by numerous groups. Compared to some of the published series, the eyes in our group IL achieved similar [21] or better results [12, 22]. Similarly, the eyes in our groups F and Z achieved similar [23,24,25] or better [26, 27] results than those reported in previous series.
Table 4 summarizes previous publications that report results with two or more of the microkeratomes that were studied in the current paper [3, 4, 6,7,8,9, 17,18,19,20, 28,29,30,31,32,33,34]. The disparity of results presented in Table 4 can be explained by the fact that numerous parameters (magnitude of ametropia treated, study design, length of follow-up, version of the femtosecond device, excimer laser used, etc.) can affect final refractive outcomes. Consequently, it is precarious to draw conclusions on the performance of different platforms from studies with different methodologies and patient populations. Our study allows a more accurate comparison of the devices as a number of biases were avoided, because all operations were performed by two experienced refractive surgeons following the same surgical protocol with the same excimer laser in operating rooms with identical temperature and humidity levels [35, 36]. Additionally, all patients received an identical postoperative eyedrop regimen. Although one limitation of this study could be the small, but statistically significant differences found in preoperative CDVA among groups, spherical and cylindrical refraction was matched within 0.5 D in order to minimize bias. To avoid the recruitment of participants with presbyopia and its influence on postoperative refractive and visual outcomes, only patients younger than 40 years were included. As far as the learning curve is concerned, all the laser platforms remained in the facilities for at least 1 year, and data from surgeries performed in the first 3 months of use of all devices were not included.
In addition to using the same excimer laser in all surgeries, we were able to obtain a more accurate description of each microkeratome’s results during the first three postoperative months. While group Z provided the most homogeneous UDVA throughout the follow-up visits and reached better CDVA at the 3-month visit, group F provided higher disparity of the results, being those that showed higher standard deviation in all the parameters studied, except in residual cylinder. Eyes in group H surpassed group IL’s results in terms of CDVA, UDVA, residual sphere, and cylinder, but similar safety and efficacy index were found between them.
We can only speculate about the reasons explaining the slower improvement of visual acuity in eyes of group F. An initial in vitro study with the device that we used in group F found that the corneal flap thickness deviation was only ± 10 μm [37], but later studies reported greater deviations [38]. These variations might be related to transient tissue changes induced by the laser treatment, such as different degrees of flap or interface inflammation and/or edema, or ultrastructural changes in the stromal bed or flap not previously described, which may vary among different units of the same device.
One limitation of the current study is that a comparison of higher-order aberrations between the platforms was not performed because such aberrometric data were unavailable in a significant proportion of participants. Such a comparison could be useful, as it could potentially discriminate safer devices for longer follow-up periods.
The current study has highlighted interesting differences in the postoperative evolution of refractive characteristics in eyes treated with various microkeratomes. Further studies are warranted to better evaluate these particular clinical and refractive characteristics with different devices, as this knowledge could be valuable for the optimization of femtosecond technology. The opinion of the surgeon regarding the ease or the difficulty in performing surgery with the different devices, and the patient perception of the surgery were not studied.
Conclusions
Compared with the other microkeratomes, a transient delay in visual restoration after LASIK surgery with the femtosecond device used in group F of our study might be expected in the early follow-up period.
References
Zhang ZH, Jin HY, Suo Y, et al. Femtosecond laser versus mechanical microkeratome laser in situ keratomileusis for myopia: metaanalysis of randomized controlled trials. J Cataract Refract Surg. 2011;37(12):2151–9.
Chen S, Feng Y, Stojanovic A, et al. IntraLase femtosecond laser vs mechanical microkeratomes in LASIK for myopia: a systematic review and meta-analysis. J Refract Surg. 2012;28(1):15–24.
Moshirfar M, Gardiner JP, Schliesser JA, et al. Laser in situ keratomileusis flap complications using mechanical microkeratome versus femtosecond laser: retrospective comparison. J Cataract Refract Surg. 2010;36(11):1925–33.
Kezirian GM, Stonecipher KG. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J Cataract Refract Surg. 2004;30(4):804–11.
Farjo AA, Sugar A, Schallhorn SC, et al. Femtosecond lasers for LASIK flap creation: a report by the American Academy of Ophthalmology. Ophthalmology. 2013;120(3):e5–20.
Chan A, Ou J, Manche EE. Comparison of the femtosecond laser and mechanical keratome for laser in situ keratomileusis. Arch Ophthalmol. 2008;126(11):1484–90.
Medeiros FW, Stapleton WM, Hammel J, et al. Wavefront analysis comparison of LASIK outcomes with the femtosecond laser and mechanical microkeratomes. J Refract Surg. 2007;23(9):880–7.
Tran DB, Sarayba MA, Bor Z, et al. Randomized prospective clinical study comparing induced aberrations with IntraLase and Hansatome flap creation in fellow eyes: potential impact on wavefront-guided laser in situ keratomileusis. J Cataract Refract Surg. 2005;31(1):97–105.
Durrie DS, Kezirian GM. Femtosecond laser versus mechanical keratome flaps in wavefront-guided laser in situ keratomileusis: prospective contralateral eye study. J Cataract Refract Surg. 2005;31(1):120–6.
Salomao MQ, Ambrosio R Jr, Wilson SE. Dry eye associated with laser in situ keratomileusis: mechanical microkeratome versus femtosecond laser. J Cataract Refract Surg. 2009;35(10):1756–60.
Knorz MC, Vossmerbaeumer U. Comparison of flap adhesion strength using the Amadeus microkeratome and the IntraLase iFS femtosecond laser in rabbits. J Refract Surg. 2008;24(9):875–8.
Sanchez-Pina JM, Arranz-Marquez E, Gil Ciganda N, et al. LASIK results of IntraLase femtosecond laser to correct myopia: efficacy, security and predictability. Arch Soc Esp Oftalmol. 2007;82(7):423–8.
Ahn H, Kim JK, Kim CK, et al. Comparison of laser in situ keratomileusis flaps created by 3 femtosecond lasers and a microkeratome. J Cataract Refract Surg. 2011;37(2):349–57.
Vetter JM, Holzer MP, Teping C, et al. Intraocular pressure during corneal flap preparation: comparison among four femtosecond lasers in porcine eyes. J Refract Surg. 2011;27(6):427–33.
Zheng Y, Zhou Y, Zhang J, et al. Comparison of laser in situ keratomileusis flaps created by 2 femtosecond lasers. Cornea. 2015;34(3):328–33.
Huhtala A, Pietilä J, Mäkinen P, et al. Femtosecond lasers for laser in situ keratomileusis: a systematic review and metaanalysis. Clin Ophthalmol. 2016;10:393–404.
Lim T, Yang S, Kim M, et al. Comparison of the IntraLase femtosecond laser and mechanical microkeratome for laser in situ keratomileusis. Am J Ophthalmol. 2006;141(5):833–9.
Patel SV, Maguire LJ, McLaren JW, et al. Femtosecond laser versus mechanical microkeratome for LASIK; a randomized controlled study. Ophthalmology. 2007;114(8):1482–90.
Rosa AM, Neto Murta J, Quadrado MJ, et al. Femtosecond laser versus mechanical microkeratomes for flap creation in laser in situ keratomileusis and effect of postoperative measurement interval on estimated femtosecond flap thickness. J Cataract Refract Surg. 2009;35(5):833–8.
Calvo R, McLaren JW, Hodge DO, et al. Corneal aberrations and visual acuity after laser in situ keratomileusis: femtosecond laser versus mechanical microkeratome. Am J Ophthalmol. 2010;149(5):785–93.
Yu CQ, Manche EE. Comparison of 2 femtosecond lasers for flap creation in myopic laser in situ keratomileusis: one-year results. J Cataract Refract Surg. 2015;41(4):740–8.
Alio JL, Vega-Estrada A, Piñero DP. Laser-assisted in situ keratomileusis in high levels of myopia with the amaris excimer laser using optimized aspherical profiles. Am J Ophthalmol. 2011;152(6):954–63.
Winkler von Mohrenfels C, Khoramnia R, Salgado J, et al. First clinical results with a new 200 kHz femtosecond laser system. Br J Ophthalmol. 2012;96(6):788–92.
Kanellopoulos AJ, Asimellis G. Long-term bladeless LASIK outcomes with the FS200 Femtosecond and EX500 Excimer Laser workstation: the Refractive Suite. Clin Ophthalmol. 2013;7:261–9.
Durrie DS, Brinton JP, Avila MR, et al. Evaluating the speed of visual recovery following thin-flap LASIK with a femtosecond laser. J Refract Surg. 2012;28(9):620–4.
Au JD, Krueger RR. Optimized femto-LASIK maintains preexisting spherical aberration independent of refractive error. J Refract Surg. 2012;28:S821–5.
Pietilä J, Huhtala A, Mäkinen P, et al. Laser-assisted in situ keratomileusis flap creation with the three-dimensional, transportable Ziemer FEMTO LDV model Z6 I femtosecond laser. Acta Ophthalmol. 2014;92(7):650–5.
Shetty R, Malhotra C, D’Souza S, et al. WaveLight FS200 vs Hansatome LASIK: intraoperative determination of flap characteristics and predictability by hand-held bioptigen spectral domain ophthalmic imaging system. J Refract Surg. 2012;28(11 suppl):S815–20.
Zhang XX, Zhong XW, Wu JS, et al. Corneal flap morphological analysis using anterior segment optical coherence tomography in laser in situ keratomileusis with femtosecond lasers versus mechanical microkeratome. Int J Ophthalmol. 2012;5(1):69–73.
Hasimoto AR, Gomes MF, de Siqueira MA, et al. Femtosecond laser versus mechanical microkeratome for LASIK flap creation. Arq Bras Oftalmol. 2013;76(6):335–8.
Liu Q, Zhou YH, Zhang J, et al. Comparison of corneal flaps created by Wavelight FS200 and Intralase FS60 femtosecond lasers. Int J Ophthalmol. 2016;9(7):1006–10.
Meidani A, Tzavara C. Comparison of efficacy, safety, and predictability of laser in situ keratomileusis using two laser suites. Clin Ophthalmol. 2016;10:1639–46.
Tomita M, Chiba A, Matsuda J, et al. Evaluation of LASIK treatment with the Femto LDV in patients with corneal opacity. J Refract Surg. 2012;28(1):25–30.
Tomita M, Sotoyama Y, Yukawa S, et al. Comparison of DLK incidence after laser in situ keratomileusis associated with two femtosecond lasers: Femto LDV and IntraLase FS60. Clin Ophthalmol. 2013;7:1365–71.
Walter KA, Stevenson AW. Effect of environmental factors on myopic LASIK enhancement rates. J Cataract Refract Surg. 2004;30(4):798–803.
Dantas PE, Martins CL, de Souza LB, et al. Do environmental factors influence excimer laser pulse fluence and efficacy? J Refract Surg. 2007;23(3):307–9.
Mrochen M, Wüllner C, Krause J, et al. Technical aspects of the WaveLight FS200 femtosecond laser. J Refract Surg. 2010;26(10):S833–40.
Cummings AB, Cummings BK, Kelly GE. Predictability of corneal flap thickness in laser in situ keratomileusis using a 200 kHz femtosecond laser. J Cataract Refract Surg. 2013;39(3):378–85.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization—Miguel A. Teus, Juan Gros-Otero, Isabel Rodriguez-Perez. Methodology—Miguel A. Teus, Juan Gros-Otero, Isabel Rodriguez-Perez, Montserrat Garcia-Gonzalez, Andreas Katsanos, Dimitrios G. Mikropoulos. Formal analysis and investigation—Miguel A. Teus, Juan Gros-Otero, Isabel Rodriguez-Perez. Writing—original draft preparation—Juan Gros-Otero, Isabel Rodriguez-Perez, Andreas Katsanos. Writing, review and editing—Miguel A. Teus, Juan Gros-Otero, Isabel Rodriguez-Perez, Montserrat Garcia-Gonzalez, Andreas Katsanos, Dimitrios G. Mikropoulos. Supervision—Miguel A. Teus, Montserrat Garcia-Gonzalez.
Prior Presentation
This study was presented at the American Academy of Ophthalmology annual congress (AAO) on the scientific poster/refractive surgery session: PO173. New Orleans 2017.
Disclosures
Juan Gros-Otero, Isabel Rodriguez-Perez, Miguel A Teus, Andreas Katsanos, Dimitrios G. Mikropoulos and Montserrat Garcia-Gonzalez declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
All patients provided written informed consent, and institutional review board approval was obtained (regional committee of clinical research of the Community of Madrid. REF 216/3). The study was performed in accordance with the tenets of the Declaration of Helsinki.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gros-Otero, J., Rodríguez-Pérez, I., Teus, M.A. et al. Myopic LASIK Outcomes: Comparison of Three Different Femtosecond Lasers and a Mechanical Microkeratome Using the Same Excimer Laser. Ophthalmol Ther 11, 1047–1066 (2022). https://doi.org/10.1007/s40123-022-00486-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00486-y