Abstract
Introduction
The fluocinolone acetonide (FAc) intravitreal drug-delivery system implant is a recent, second-line, intravitreal drug for the management of diabetic macular edema (DME). FAc acts against DME with a major anti-inflammatory effect. Despite the already proved efficacy, a number of patients still show persistent DME and require anti-VEGF retreatment. The main aim of the present study was to assess the relationship between quantitative biomarkers of inflammation and both DME recovery and the need for additional anti-VEGF in eyes treated by FAc implant.
Methods
The study was designed as prospective and interventional with 1 year of follow-up. We analyzed structural optical coherence tomography (OCT) quantitative biomarkers of inflammation, namely choroidal hyperreflective foci (HF) and the choroidal vascularity index (CVI), and we assessed the relationship with other clinically relevant biomarkers and the outcome achieved after 1 year. Moreover, we stratified DME eyes in good and poor responders to FAc implant to highlight clinically relevant differences.
Results
Our study included 50 eyes (50 patients) treated by FAc implant. We found significant best-corrected visual acuity (BCVA) and central macular thickness (CMT) improvements after 1 year. Good responders started with worse visual acuity and higher CMT than poor responders, but gained letters significantly at the end of the follow-up, whereas poor responders showed stable BCVA values. Good responders were characterized by significantly higher choroidal HF and lower CVI than poor responders. Poor responders required significantly higher additional anti-VEGF treatments.
Conclusions
Quantitative structural OCT biomarkers of inflammation allowed distinguishing different inflammatory profiles of DME. The inflammatory component helped to categorize DME eyes in good and poor responders to FAc implant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Diabetic macular edema pathogenesis has inflammation and vasculopathy as key factors. The different contribution of these two elements may justify the heterogeneous response to anti-VEGF and intravitreal steroid treatments. |
Fluocinolone acetonide (FAc) intravitreal drug-delivery system implant is a recent second-line treatment for the long-term management of DME. |
In the present study, we evaluated the relationship between quantitative structural OCT biomarkers of inflammation and the response to FAc implant. |
We quantitatively categorize DME eyes, accordingly to the inflammatory profiles, in good and poor responders to FAc implant. |
We proposed quantitative cutoff values for the selection of good DME candidates for FAc implant. |
Digital Features
This article is published with digital features to facilitate understanding of the article. To view digital features for this article go tohttps://doi.org/10.6084/m9.figshare.12860672.
Introduction
Diabetic macular edema (DME) represents a possible complication of diabetic retinopathy (DR), leading to progressive visual acuity loss [1, 2]. The current treatments include both intravitreal anti-VEGF injections and corticosteroids [3]. The two main issues related to DME management are the heterogeneous response to each intravitreal drug and the need for even more long-term duration treatments. More recently, the fluocinolone acetonide (FAc) 0.19 mg intravitreal drug-delivery system (ILUVIEN®; Alimera Sciences, Inc., Alpharetta, GA, USA) has been introduced to provide a safe and efficient long-term therapeutic approach, with up to 3-year duration [4,5,6,7,8,9]. Since the FAc implant mainly has an anti-inflammatory mechanism of action, the variable response to this specific treatment may depend on the amount of inflammation characterizing DME.
The main aim of the present study was to quantitatively investigate retinal morphologic features in DME patients treated by FAc implant to assess the presence of quantitative structural optical coherence tomography (OCT) biomarkers related to good response to FAc treatment.
The secondary goal was the identification of two different subgroups of DME eyes according to the quantitative inflammatory profiles detected by structural OCT.
Methods
The study was designed as a prospective, interventional cohort study including DME eyes treated with the FAc implant at the Department of Ophthalmology, San Raffaele Hospital, Milan, Italy. The study was approved by the the ethics committee of San Raffaele Hospital, Milan, Italy, and was conducted in accordance with Helsinki Declaration. All the patients signed an informed consent before inclusion in the study.
The inclusion criterion was the presence of DME treated with an intravitreal FAc implant, with at least 1 year of follow-up. The administration of the FAc implant was caried out in accordance with the Italian guidelines, comprising refractory DME in pseudophakic patients. Exclusion criteria consisted of macular edema secondary to other causes than DME, high media opacities, any ophthalmic surgery in the last 6 months before FAc implant, uncontrolled glaucoma or any ophthalmic or systemic disease potentially affecting the results of the study. All the patients underwent complete ophthalmologic examination including ETDRS best corrected visual acuity (BCVA), anterior and posterior segment slit-lamp evaluation and Goldmann applanation tonometry. We performed structural OCT examinations (Spectralis HRA, Heidelberg Engineering; Heidelberg, Germany) with radial, raster and dense scans with a high number of frames (ART > 25). Enhanced depth imaging (EDI) was applied to highlight choroidal structures. From structural OCT images, we measured the following parameters: central macular thickness (CMT), inner retinal thickness (IRT), outer retinal thickness (ORT), disorganization of the inner retinal layers (DRIL), epiretinal membrane (ERM), ellipsoid zone (EZ) and external limiting membrane (ELM) status, retinal and choroidal hyperreflective foci (HF), subretinal fluid (SRF) and choroidal thickness (CT). With respect to the HF calculation, to make the HF quantification clinically feasible, we arbitrarily considered a number of macular HF > 15 positive, considering retinal and choroidal HF separately.
Moreover, we calculated the choroidal vascularity index (CVI) parameter starting from a horizontal high-resolution structural OCT scan. We loaded the images in ImageJ software [10]. Then, two expert ophthalmologists (AA, LC) segmented the choroid, excluding those regions affected by reflectivity hyper-transmission secondary to retinal pigmented epithelium damage. They also excluded all the rest of the retina and binarized the choroidal images, applying a mean threshold. In this way, choroidal vessels were black whereas choroidal stroma white. Lastly, in-house scripts were used to calculate the ratio between white and black signals, thus obtaining the final CVI value.
The main outcome measure of this study was the assessment of the relationship between choroidal parameters and the final visual and anatomical outcome after 1 year of follow-up. To achieve this goal, we stratified patients into good and poor responders at the end of the 1-year follow-up. The a priori arbitrary criterion adopted to define good responders was the improvement of at least 30% of the CMT. For the analysis, we considered baseline (the last visit before FAc implant) and 1-year examinations. The interclass correlation coefficient (ICC) was measured to assess the agreement between the two graders through a two-way random-effects model. Age, gender, systemic hypertension, type and duration of diabetes mellitus (DM), glycate hemoglobin (HbA1c), stage of DR, duration of DME, previous history of vitrectomy, previous panretinal photocoagulation (PRP), previous nature and number of treatments (anti-VEGF, intravitreal corticosteroids), baseline features (BCVA and CMT), retinal and/or choroidal HF > 15, ERM, DRIL, ELM/EZ status (normal/interrupted/absent) and SRF were considered fixed factors. We also considered the eventual presence of additional anti-VEGF treatment (yes/no) administered during the follow-up in accordance with ophthalmologists’ discretion for persistent DME. All the statistical analyses were performed using the SPSS software package (SPSS, Chicago, IL, USA), through univariate and multivariate analyses, with Bonferroni correction applied to assess multiple comparisons. Kendall tau correlation analysis was adopted to assess the relationship among the included parameters. Statistical significance was set at p < 0.05.
Results
We collected data from 50 eyes of 50 patients (29 males; mean age 68 ± 9 years) affected by DME. All the eyes were pseudophakic and had been previously treated with intravitreal dexamethasone implants. All the patients were followed for a least 1 year. Clinical data are extensively reported in Table 1. In particular, we found a slightly significant BCVA improvement after 1 year (p = 0.03) together with a significant reduction of the macular edema (p < 0.01).
The overall inter-grader agreement was 93% (range 89–98%) ICC.
The post-hoc analysis between poor and good responders highlighted statistically significant clinical differences.
Good responders started with worse visual acuity than poor responders (p = 0.03), but significantly gained letters at the end of the follow-up (p = 0.03), whereas poor responders maintained stable BCVA values.
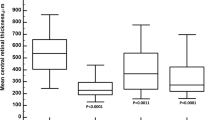
CMT results were significantly higher in good responders at baseline compared to poor responders (p = 0.02) and showed a significant macular edema regression at the end of the follow-up (p < 0.01); contrarily, poor responders showed uneven reductions of the CMT (p > 0.05).
With respect to the choroidal biomarkers, namely choroidal HF and CVI, good responders showed significantly higher choroidal HF and lower CVI than poor responders (p < 0.01), both at baseline and at 1-year follow-up. Both parameters disclosed no significant changes in poor responders over the follow-up (p > 0.05); good responders showed only the CVI increment, registered at the end of the follow-up (p < 0.01). On the other hand, CT was always similar between good and poor responders (p > 0.05) (Fig. 1).
Representative cases of good and poor responders to FAc implant. A good anatomical responder case showing low CVI at baseline (a) and showing a complete recovery of the foveal profile after 1 year (b), with just some intraretinal residual small cysts. A poor responder case showing high CVI at baseline (c) and almost unchanged DME features after 1 year of treatment (d)
Interestingly, a mean CVI value of 0.40 helped to identify good and poor responders to the FAc implant.
DRIL and SRF were similar between the two groups, both at baseline and at 1-year follow-up (p > 0.05).
Good responders showed higher baseline IRT (p = 0.02), resulting in recovery after 1 year (p < 0.01), but was unchanged in poor responders (p > 0.05). ORT and ELM/EZ status was always similar in both groups, with unremarkable changes detected at the end of the follow-up (p > 0.05).
The mean number of additional anti-VEGF injections was 2 ± 1. Poor responders underwent more additional anti-VEGF treatments than good responders in terms of the number of eyes treated (p = 0.02). We found no statistical difference in terms of the number of additional anti-VEGF injections between good and poor responders (p > 0.05).
All the values are extensively described in Table 2.
The correlation analysis (Table 3) highlighted the strict relationship between choroidal inflammatory status (characterized by low CVI and high choroidal HF number) and the good response to the FAc implant. Additional anti-VEGF treatments were only related to the final CMT values.
Discussion
In the present study, we evaluated the impact of structural OCT biomarkers of inflammation on the response to FAc implant over 1 year of follow-up. The stratification of good and poor responders to this intravitreal corticosteroid treatment, based on the anatomical outcome (DME reduction) achieved after 1 year, highlighted two important features.
First, good responders were characterized by significantly higher inflammatory biomarkers together with a significantly higher amount of macular edema at baseline. This means that the presence of a pronounced inflammatory profile at baseline, assessed through a quantitative analysis, is associated to a greater efficacy of intravitreal corticosteroids. Second, the functional recovery after 1 year of FAc activity was remarkably better in good responders than in poor responders, as shown by the BCVA and CMT improvements.
It is worth noting that poor responders globally maintained stable CMT and BCVA values at the end of the follow-up, thus indicating FAc treatment as a feasible treatment also in DME eyes characterized by less pronounced inflammatory profiles.
It is well known that the pathogenesis of DR and DME is extremely complex and involves different metabolic pathways and retinal cytotypes [11, 12]. The two faces of the DME coin are represented by inflammation and vasculopathy [11, 12]. Although present at the same time, it can be assumed that different DME eyes might disclose different inflammatory and vasculopathy contributions. The different involvement of these two pathogenic factors represents a relevant basis to understand the heterogeneous DME response to different intravitreal drugs.
Both anti-VEGF and corticosteroids were efficient for the management of DME [3]. The more recently introduced FAc implant addressed important issues related to the treatment of DME patients, i.e., the target of chorioretinal inflammation pathways and the frequency of retreatments. However, as highlighted by previous studies [13,14,15], also in the case of the FAc implant, the proper patient selection is fundamental to normalizing the heterogeneity of the treatment response and to reaching the therapeutic goal.
In this context, structural OCT offered the opportunity to evaluate useful biomarkers in a non-invasive way. Indeed, this technique highlighted how heterogeneous DME can be in terms of retinal morphology and coexisting alterations [16, 17].
Choroidal OCT biomarkers, including HF and CVI, have already been proposed as feasible and useful metrics for the evaluation of DR and DME and for their monitoring over the follow-up [18, 19]. Although both parameters may be related to inflammation, their meaning is quite different.
HFs are commonly interpreted as inflammatory cell aggregates, affecting both the retina and the choroid [18]; CVI, intended as the ratio between the choroidal stroma and vessels, provides information regarding choroidal vessel status and may be considered a biomarker of choroidal congestion [20, 21].
In agreement with these previous interpretations of HF and CVI, the presence of a high number of choroidal HF and low CVI values, this latter representing intense choroidal congestion, can be interpreted as structural OCT signs of increased chorioretinal inflammation [22]. From this perspective, the categorization of DME eyes according to these structural OCT biomarkers of inflammation may have a role when making the proper choice of intravitreal therapeutic strategy in the DME setting.
In our study, the combined presence of a high number of choroidal HF and low CVI values was strongly associated with higher efficacy of the FAc implant.
It is worth noting that a CVI value of 0.4 characterized good and poor responders’ eyes, thus suggesting this value as a possible cutoff to be evaluated in future larger prospective studies.
Although CVI was associated with the response to the FAc implant, it showed no relationship with CT, thus reinforcing the hypothesis of a greater specificity of CVI than CT in evaluating the choroidal inflammatory status [23].
With respect to HF, our findings showed that, whereas choroidal HF was subjected to relevant changes over the follow-up, intraretinal HF was similar between good and poor responders’ eyes at baseline, and their values remained stable over the follow-up. This might suggest that these lesions are linked to different pathogenic mechanisms with respect to choroidal HF. Further studies are warranted to better clarify the clinical meaning of retinal and choroidal HF.
The other evaluated parameters, namely DRIL, ELM/EZ status and SRF, were always similar between the two DME groups, thus suggesting the leading pathogenic mechanisms were not linked with the chorioretinal inflammatory status.
The additional administration of anti-VEGF was significantly more frequent in the poor responder group, and the only parameter related with the choice to retreat was the CMT. The influence of the CMT on the clinical choice to retreat DME eyes in the FAc implant setting has been recently highlighted by Cicinelli and colleagues and might somehow be expected [15]. To date, the choice to perform additional treatments is mainly made according to the ophthalmologist's discretion, and it has extremely heterogeneous clinical efficacy. This further reinforces the need for even more specific quantitative biomarkers for the prediction of the need to retreat patients after FAc implant.
We are aware that our study has several possible limitations. The first may be the lack of DME eyes treated with other intravitreal drugs (anti-VEGF and dexamethasone implant). We made the choice to include only DME eyes treated with the FAc implant to reduce the variability related to the different treatment choices. The inclusion of DME eyes treated with the FAc implant guaranteed a stable drug concentration and then a reliable evaluation of quantitative biomarker changes over the follow-up. Furthermore, with respect to the HF quantification, we arbitrarily adopted a cutoff of HF > 15 in the attempt to make it feasible for the readers to apply this approach in common clinical settings. However, we are aware that further investigations of HF number changes are warranted to assess the role of this quantitative biomarker in the evaluation of FAc efficacy in depth. Although we limited our observations to 1 year, even though the declared FAc duration is 3 years, we believe this follow-up may be considered enough to assess the inflammatory biomarker changes secondary to the FAc implant; however, our study should just be considered a first investigation paving the way for future larger studies. However, we acknowledge that the low number of eyes and the relatively short follow-up make our study just an exploratory investigation of the role of quantitative OCT biomarkers of inflammation in the assessment of the FAc implant's effect in DME. For this reason, our findings will benefit from further validations provided by larger prospective studies.
Conclusion
In conclusion, our study highlighted how the evaluation of structural OCT biomarkers of inflammation may be useful to categorize DME eyes in good and poor responders to FAc implants, according to the quantitatively measured chorioretinal inflammatory profile. Independently from this kind of stratification, the FAc implant was found a feasible and useful treatment for the management of DME. From the perspective of even more optimized and personalized treatment strategies, further studies are warranted to identify specific quantitative cutoff values for treating DME eyes with anti-VEGF injections or corticosteroid implants.
References
Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122(7):1375–94.
Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R, Verges R. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res. 2016;2016:2156273.
Schmidt-Erfurth U, et al. Guidelines for the management of diabetic macular edema by the european society of retina specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222.
Campochiaro PA, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–32.
Campochiaro, P.A., et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2012;118(4):626–635e622.
Alfaqawi F, Lip PL, Elsherbiny S, Chavan R, Mitra A, Mushtaq B. Report of 12-months efficacy and safety of intravitreal fluocinolone acetonide implant for the treatment of chronic diabetic macular oedema: a real-world result in the United Kingdom. Eye (Lond). 2017;31(4):650–6.
Fusi-Rubiano W, et al. Treating diabetic macular oedema (DMO): real world UK clinical outcomes for the 0.19 mg fluocinolone acetonide intravitreal implant (Iluvien) at 2 years. BMC Ophthalmol. 2018;18(1):62.
Bailey C, Chakravarthy U, Lotery A, Menon G, Talks J, Medisoft Audit G. Real-world experience with 0.2 mug/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye (Lond). 2017;31(12):1707–15.
Augustin AJ, et al. Three-year results from the Retro-IDEAL study: Realworld data from diabetic macular edema (DME) patients treated with ILUVIEN((R)) (0.19 mg fluocinolone acetonide implant). Eur J Ophthalmol. 2019;30(2):382–91.
Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82.
Tan GS, Cheung N, Simó R, Cheung GC, Wong TY. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017;5(2):143–55.
Chakravarthy U, et al. Clinical evidence of the multifactorial nature of diabetic macular edema. Retina. 2018;38(2):343–51.
Young JF, Walkden A, Stone A, Mahmood S. Clinical effectiveness of intravitreal fluocinolone acetonide (FAc) (ILUVIEN™) in patients with diabetic macular oedema (DMO) refractory to prior therapy: the manchester experience. Ophthalmol Ther. 2019;8(3):477–84.
Holden SE, Habib M, Currie CJ. Retinal thickness fluctuations in patients receiving fluocinolone acetonide implant for diabetic macular edema. Curr Med Res Opin. 2020;1–7. [Epub ahead of print].
Cicinelli MV, Rabiolo A, Zollet P, Capone L, Lattanzio R, Bandello F. Persistent or recurrent diabetic macular edema after fluocinolone acetonide 0.19 mg Implant: risk factors and management. Am J Ophthalmol. 2020. [Epub ahead of print].
Parodi Battaglia M, Iacono P, Cascavilla M, Zucchiatti I, Bandello F. A pathogenetic classification of diabetic macular edema. Ophthalmic Res. 2018;60(1):23–8.
Panozzo G, et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: the European school for advanced studies in ophthalmology classification. Eur J Ophthalmol. 2020;30(1):8–18.
Schreur V, et al. Hyperreflective foci on optical coherence tomography associate with treatment outcome for anti-VEGF in patients with diabetic macular edema. PLoS ONE. 2018;13(10):e0206482.
Gupta C, et al. Choroidal structural analysis in eyes with diabetic retinopathy and diabetic macular edema-A novel OCT based imaging biomarker. PLoS ONE. 2018;13(12):e0207435.
Wei X, Ting DSW, Ng WY, Khandelwal N, Agrawal R, Cheung CMG. CHOROIDAL VASCULARITY INDEX: a novel optical coherence tomography based parameter in patients with exudative age-related macular degeneration. Retina. 2017;37(6):1120–5.
Kim M, Ha MJ, Choi SY, Park YH. Choroidal vascularity index in type-2 diabetes analyzed by swept-source optical coherence tomography. Sci Rep. 2018;8(1):70.
Iovino C, Pellegrini M, Bernabei F, et al. Choroidal vascularity index: an in-depth analysis of this novel optical coherence tomography parameter. J Clin Med. 2020;9(2):595.
Tan KA, Laude A, Yip V, Loo E, Wong EP, Agrawal R. Choroidal vascularity index—a novel optical coherence tomography parameter for disease monitoring in diabetes mellitus? Acta Ophthalmol. 2016;94(7):e612–e616616.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Francesco Bandello is a consultant for: Alcon (Fort Worth, Texas, USA), Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California, USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, New York, USA), Genentech (San Francisco, California, USA), Hoffmann-La-Roche (Basel, Switzerland), NovagaliPharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA). Alessandro Arrigo, Luigi Capone, Rosangela Lattanzio, Emanuela Aragona and Piero Zollet have no disclosures to declare.
Compliance with Ethics Guidelines
The study was approved by the the ethical committee of San Raffaele Hospital, Milan, Italy, and was conducted in accordance with the Helsinki Declaration. All the patients signed an informed consent before the inclusion into the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12860672
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Arrigo, A., Capone, L., Lattanzio, R. et al. Optical Coherence Tomography Biomarkers of Inflammation in Diabetic Macular Edema Treated by Fluocinolone Acetonide Intravitreal Drug-Delivery System Implant. Ophthalmol Ther 9, 971–980 (2020). https://doi.org/10.1007/s40123-020-00297-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-020-00297-z