Abstract
Background
Previous research highlights burning eye syndrome (BES) and burning mouth syndrome (BMS) as chronic complications of COVID-19 infection. The aim of this systematic review and meta-analysis is to establish the prevalence of COVID-19-related BES and COVID-19-related BMS and describe their phenomenology.
Methodology
A literature search in the PubMed database was performed, and seven papers (five on BES and two on BMS) were eligible to be included in this systematic review and meta-analysis.
Results
The pooled prevalence of COVID-19-related BES was 9.9% (95% CI 3.4–25.4%). The frequency of COVID-19-related BMS is only reported in isolated cases and ranges from 4% in mild-to-moderate cases to 15% in severe, hospitalized cases, with female patients being mostly affected. COVID-19 severity is a potential risk factor for both BES and BMS. Neither syndrome occurs in isolation. COVID-19-related BES usually appears within the first week post-infection, persisting up to 9 weeks later. COVID-19-related BMS occurs during and after initial infection, and may also persist as a chronic disease.
Conclusions
Both BES and BMS are neuropathic COVID-19 infection complications, still under-studied and under-investigated, despite the fact that both are prevalent. Both COVID-19-related BES and COVID-19-related BMS could potentially be initial long COVID syndrome manifestations, and further research should be carried out in this field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
SARS-CoV-2, responsible for the coronavirus disease (COVID-19) pandemic [6], is a virus that may cause permanent lung damage as well as impact multiple other systems [7, 20, 23]. Among the symptoms of COVID-19, burning eyes and burning mouth are included.
Causes of burning eyes include autoimmune diseases leading to eye dryness [12, 28, 37], allergies, inflammation, and injuries [2, 3, 5]. Burning eye syndrome (BES) is considered a chronic neuropathic pain disorder [19] that occurs when patients experience ocular burning pain in the absence of inflammation or injury.
Burning mouth is generally present in a spectrum of conditions, for example, candidiasis and allergy [17]. Similar to BES, burning mouth syndrome (BMS) is considered neuropathic in origin [16], and occurs when patients experience unexplained chronic oral mucosal burning [4, 9]. Previous research proposed trigeminal neuropathy as a potential BMS cause [22].
Previous observations have highlighted the association between BES/BMS developing or worsening following COVID-19 infection (BES—[10, 27] (BMS—[9, 11, 36, 40]. Therefore, BES and BMS are suggested COVID-19 complications. However, the exact prevalence of BES and BMS following COVID-19 infection remains unestablished [1]. Biological mechanisms and how these syndromes occur, both during and after COVID-19 infection, also remain unknown to date.
This systematic review aims to establish the prevalence, determinants, and clinical characteristics of COVID-19-related BES and COVID-19-related BMS.
Methods
Protocol and Registration
This review was registered in PROSPERO, an international database of prospectively registered systematic reviews in health and social care. The registration number for this review is CRD42022330381.
Literature Search Strategy
A systematic literature search in the PubMed database was performed on 16 May 2022 using two medical subject heading terms that had to be present in the title or the abstract. Term A was “Allodynia OR hyperalgesia OR hypoalgesia OR itchiness OR burning OR neuropathic OR ‘painful cold’ OR ‘electric shock’ OR tingling OR ‘pins and needles’ OR itchiness OR itching OR hypoesthesia”, and term B was “COVID or SARS-CoV-2 or Coronavirus”. Human subjects, English language, and full-text filters were applied. The reference lists of eligible papers and relevant reviews were also meticulously searched to include further studies reporting on BES or BMS related to COVID-19.
Decisions regarding search term use involved the Population, Intervention, Comparison, Outcome (PICO) framework [29], highlighted in Table 1. The PICO framework aids in the formulation/discovery of answers to healthcare research questions in an evidence-based manner [8]. Specifically, in this review the PICO framework helped to decide how to best detect articles related to COVID-19-related BES/BMS for analysis. This helped to answer the research objectives regarding prevalence.
Inclusion/Exclusion Criteria
Articles eligible to be included in this review had to meet the following criteria: (i) papers describing patients with BES or BMS that was clearly linked to COVID-19, (ii) human adult subjects were involved, and (iii) the article was written in English.
The following comprised the exclusion criteria for this review: (i) description of BES or BMS was not within the primary aims of the study, (ii) case series/cohorts with fewer than ten patients, (iii) non-original articles (review, medical hypothesis, letter to the editor, etc.), and (iv) articles describing BES or BMS of other causes.
In descending order, title screening, abstract screening, full-text screening, and reference screening were implemented to filter out non-relevant papers. This left relevant articles suitable for inclusion. Decisions regarding which papers were eligible for inclusion were derived from the two researchers. Reference screening was implemented using Google Scholar purely to identify any papers that may have been missed during the original search. However, reference screening yielded no additional literature. This systematic review and meta-analysis is based on published papers and there are no ethical issues.
Data Extraction
For those eligible articles, information extracted included phenotypes, incidence, natural history, and treatments. This information was then collated. Additional narrative summaries were undertaken. By utilizing color coding, the content of each article was successfully assigned for use within the narrative summary sections of this review. Data regarding comorbidities, demographics, and COVID-19 severity were extracted, alongside time lengths (between initial COVID-19 infection and first presentations of COVID-19-related BES/BMS) and different neuropathy forms. Long COVID details were extracted for use within the statistical meta-analyses in this review. Full-text scrutiny allowed for COVID-19-related BES/BMS risk factor identification, contributing to the overall field.
Synthesis of Results
Extracted data in this review aided prevalence investigation. Frequencies, other determinants, and descriptive components were also informed by the data. Calculated as means, 95% confidence intervals, and meta-analysis (p < 0.05), this was in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
Statistical Analysis
Aggregate data were used where possible. Statistically pooled proportion calculations conducted in R language used default settings of the “meta” package and “metaprop” function (random effects model) [21]. Each meta-analysis presented the I2 statistic and forest plots, which evaluated heterogeneity [14]. Variation can be suggested as study heterogeneity or chance using this statistic. Negative I2 values are put equal to 0, and values range between 0% and 100% [14]. Heterogeneity can be quantified as low, moderate, and high, with upper limits of 25%, 50%, and 75% for I2, respectively [14]. Where data did not lend themselves to meta-analysis, a narrative approach was taken.
Assessment of Bias
Bias assessments adapted from Hoy et al. [15] assessed the methodological quality of the included articles via a checklist. The response rate, sample representativeness, data collection method, measures used, statistical methods, and sampling techniques were covered by nine questions. Each question was labelled as “0” or “1” (0, low risk of bias, 1, high risk of bias), and total scores ranged from 0 to 9, categorized below [24]: 7–9, “High risk of bias;” 4–6, “Moderate risk of bias;” and 0–3: “Low risk of bias.”
Supplementary material details this bias assessment.
Results
Following the systematic literature search strategy described above, 384 papers were initially identified.
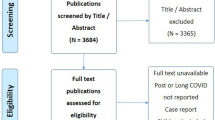
Regarding BES, 273 papers were excluded during abstract screening and 106 were excluded during full-text eligibility screening. Therefore, 5 articles met eligibility and were finally included in this review [25, 31,32,33, 39]. All included articles were observational studies. The screening and inclusion process is highlighted in Fig. 1.
Regarding BMS, 273 papers were excluded during abstract screening and 109 were excluded during full-text eligibility screening. Therefore, two articles met eligibility and were finally included in this review [26, 35]. All included articles were observational studies. The screening and inclusion process is highlighted in Fig. 2.
Epidemiology
Meta-analysis of the five available studies [25, 31,32,33, 39] providing prevalence data was performed. The pooled prevalence of BES after COVID-19 has been established to be 9.9% (95% CI 3.4–25.4%) on the basis of 1550 patients (Fig. 3). However, there was high heterogeneity across these studies (I2 = 96.2%).
Muthyam et al. [26] described the COVID-19-related BMS frequency during (4%) and after (15%) COVID-19 infection for mild-to-moderate cases. Sinjari et al. [35] described the COVID-19-related BMS frequency in severe, hospitalized cases (15%). As only two papers provided prevalence data, a meta-analysis of these figures was not attempted.
Risk Factors for BES
Demographics
Neither age nor gender significantly impacted COVID-19-related BES [25, 33, 39].
COVID-19 Severity
The majority of COVID-19-related BES articles investigated moderate-to-severe COVID-19 infection [25, 32, 33], but two additionally addressed mild COVID-19 infection [30, 39]. Findings were somewhat contradictory. The majority of the studies found no association between COVID-19-related BES and COVID-19 severity [25, 30, 33, 39]. On the contrary, one study [32] showed that COVID-19 severity and ocular symptoms were significantly associated, that is, as COVID-19 severity increases, so does ocular symptom frequency, including burning eyes.
Risk Factors for BMS
Demographics
Age and gender had no statistically significant impact on COVID-19-related BMS. However, all patients hospitalized with severe COVID-19 infection and COVID-19-related BMS were female [35].
COVID-19 Severity
The two articles describing COVID-19-related BMS acted as reasonable comparison: one addressing mild-to-moderate COVID-19 infection [26], the other severe COVID-19 infection [35]. Initially, mild-to-moderate COVID-19 cases displayed COVID-19-related BMS frequency of 4%. However, with COVID-19 severity kept constant, this frequency increased over time to 15% [26]. Coincidentally, severe COVID-19 cases displayed COVID-19-related BMS frequency of 15% [35]. Results indicate that COVID-19-related BMS increases as COVID-19 severity increases, initially. However, why COVID-19-related BMS incidence increases over time, when severity remains constant long after COVID-19 infection, cannot be explained by this review. Noteworthy, both articles [26, 35] include patient comorbidities, for example, diabetes (10% and 15%, respectively), known to increase the risk of COVID-19 severity [35] and neuropathic pain [31]. Confoundingly, this could have influenced COVID-19-related BMS incidence.
Clinical Presentation of BES
Clinical presentation for COVID-19-related BES varied [25, 31,32,33, 39]. Many patients presented with a multitude of ocular symptoms alongside COVID-19-related BES [39], including redness, watering, and itching [30]. Rokohl et al. [30] highlighted these symptoms that often appeared together. Additionally, ocular symptom presence was not associated with generalized COVID-19 symptoms (fever, cough, headache, nasal symptoms, and sore throat). Additionally, those with ocular symptoms are more likely to present with generalized eye pain during COVID-19 disease [33]. Conjunctivitis occurred as a first initial presentation of COVID-19-related BES in 1% of patients [32]. Whether COVID-19-related BES is associated with conjunctivitis is unknown.
Clinical Presentation of BMS
COVID-19-related BMS can present with mouth ulcers (10%), gum bleeding (6%), xerostomia (44%), and swallowing difficulties (16%) [26]. Of these, swallowing difficulties (2%), mouth ulcers (12%), and gum bleeding (5%) persisted [26]. Therefore, elapsed time following COVID-19 infection impacts the types of symptoms present alongside COVID-19-related BMS. Sinjari et al. [35] corroborates impaired taste (25%) and swallowing difficulty (20%) occurrence (during COVID-19 disease). Therefore, COVID-19-related BMS rarely occurs in isolation.
Natural History
COVID-19-related BES mostly occurred within 1 week of initial COVID-19 infection [25, 31,32,33]. Corroborating each other, Rokohl et al. [30] and Seghal et al. [32] discovered the time length between COVID-19 infection and ocular manifestations was 3 and 3.12 days, respectively. Vallejo-Garcia et al. [39] assessed COVID-19-related BES 3–9 weeks (median, 6) following COVID-19 infection, demonstrating that 18% still experienced COVID-19-related BES symptoms. Therefore, COVID-19-related BES is not only an acute disease, but a persisting one.
COVID-19-related BMS was significantly affected by time length. COVID-19 BMS incidence increased over the duration of COVID-19 disease (4% to 15%) [26]. Sinjari et al. [35] adopted cross-sectional study design; thus, time length conclusions were not viable.
Discussion
This review sought to establish the prevalence of COVID-19-related BES and COVID-19-related BMS. Secondary objectives sought to collectively determine any clinical characteristics of these syndromes, risk factors, and natural history of these syndromes. Almost one in ten patients with COVID-19 experienced BES in the acute phase of the disease. BMS was less frequent (4%) in mild-to-moderate cases but more frequent (15%) in severe cases in the acute phase of COVID-19.
Previous evidence highlights how BES is a chronic neuropathic pain disorder [19], prevalent among a subset of patients with dry eyes [13]. Given this, perhaps COVID-19-related BES persists for months after initial COVID-19 infection due to being a chronic pain disorder. As COVID-19-related BES appeared acutely after infection and lasted for months, it lends support to Joshi et al. [18]. Furthermore, COVID-19-related BES might be an initial manifestation of long COVID syndrome [38].
Pertinent findings related to COVID-19-related BES suggested that COVID-19 severity and increased ocular manifestation frequency are associated with COVID-19-related BES [30]. Certain patients with COVID-19 and with a multitude of eye symptoms may benefit from eye screening to diagnose and promptly treat COVID-19-related BES, especially when considering the ocular manifestations that can present themselves in the 3–7 days after COVID-19 diagnosis [30, 32]. This might aid clinical practice, meaning that any patients with COVID-19 are offered eye treatments sooner, regardless of BES status.
COVID-19-related BMS is currently an unexplored field, though this may eventually change. Comorbidity presence, oral symptom frequency, and COVID-19 severity were highlighted as significant risk factors [35]. Considering this, patients with COVID-19 and comorbidity and oral symptoms are most at risk, and might require extra vigilance by healthcare professionals, identifying, addressing, or treating oral symptoms as they arise. Interestingly, findings showed that COVID-19-related BMS incidence appeared to increase over COVID-19 disease duration [26].
Previous literature proposed COVID-19 complications as principally neurological or peripheral [1]. Noteworthy, the wider field proposed that COVID-19-related BMS has links to small fiber neuropathy (SFN) and neuropathic pain mechanisms [16, 22]. Considering SFN is a frequent COVID-19 complication [34], perhaps COVID-19-related BMS reflects an initial presentation of SFN brought on or exacerbated by COVID-19. If so, patients with oral mucosal burning following COVID-19 could benefit from SFN testing.
Our findings should, however, be interpreted with caution, given some limitations. Firstly, gender bias impacted certain samples [33], possibly preventing detectable differences from being uncovered. Future replications require equally balanced samples. Secondly, most studies were lacking control groups. Moreover, data about symptoms relied on self-reporting in many cases, raising the odds for recall bias. Furthermore, although it has been shown by Fernández-de-Las-Peñas et al. that different variants may lead to different symptoms, especially as part of long COVID syndrome, [41], we could not comment on whether infection by different variants has a different risk for developing BES or BMS, as such information was not available. Finally, publication bias can occur in systematic reviews and may undermine the validity of the results. This is reflected in the significant heterogeneity among the studies in our meta-analysis.
Both BES and BMS are neuropathic COVID-19 infection complications, still under-studied and under-investigated [18] despite the fact that both are prevalent. Both COVID-19-related BES and COVID-19-related BMS could potentially be initial long COVID syndrome manifestations and further research should be carried out in this field.
Conflicts of Interest
There are no conflicts of interest for this paper. None of the authors exhibit any competing interests.
References
Attal N, Martinez V, Bouhassira D. Potential for increased prevalence of neuropathic pain after the COVID-19 pandemic. Pain Rep. 2021;6(1):e884.
Barkana Y, Belkin M. Laser eye injuries. Surv Ophthalmol. 2000;44(6):459–78.
Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J. What causes eye pain? Curr Ophthalmol Rep. 2015;3(2):111–21.
Bender SD. Burning mouth syndrome. Dent Clin North Am. 2018;62(4):585–96. https://doi.org/10.1016/j.cden.2018.05.006. (Epub 2018 Jul 27 PMID: 30189984).
Berdy GJ, Hedqvist B. Ocular allergic disorders and dry eye disease: associations, diagnostic dilemmas, and management. Acta Ophthalmologica Scandinavica-Supplementum. 2000;230:32–7.
Ciotti M, Ciccozzi M, Terrinoni A, Jiang WC, Wang CB, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57(6):365–88.
Clauw DJ, Häuser W, Cohen SP, Fitzcharles MA. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain. 2020;161(8):1694.
Davies KS. Formulating the evidence-based practice question: a review of the frameworks. Evid Based Libr Inf Pract. 2011;6(2):75–80.
Dugan C, Popescu BO, Parlatescu I, Dobre M, Milanesi E, Popa C. Clinical and psychological impact of SARS-CoV-2 infection in burning-mouth syndrome patients: a comparative study. Romanian J Oral Rehabilit. 2022;14:15–25.
Feng Y, Armenti ST, Albin OR, Mian SI. Novel case of an adult with toxic shock syndrome following COVID-19 infection. Am J Ophthalmol Case Rep. 2020;20: 100843.
Ferdeghini C, Mirabelli L, Bianco E, Hari S, Maddalone M. Oral manifestations in hospitalized COVID patients. World J Dent. 2022;13(5):434–40.
Fox PC. Autoimmune diseases and Sjögren’s syndrome: an autoimmune exocrinopathy. Ann NY Acad Sci. 2007;1098(1):15–21.
Giacomazzi S, Urits I, Hoyt B, Hubble A, Cornett EM, Gress K, Charipova K, Berger AA, Kassem H, Kaye AD, Viswanath O. Comprehensive review and update of burning eye syndrome. J Patient-Centered Res Rev. 2021;8(3):255.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–9.
Jääskeläinen SK. Is burning mouth syndrome a neuropathic pain condition? Pain. 2018;159(3):610–3.
Jääskeläinen SK, Woda A. Burning mouth syndrome. Cephalalgia. 2017;37(7):627–47.
Joshi D, Gyanpuri V, Pathak A, Chaurasia RN, Mishra VN, Kumar A, Singh VK, Dhiman NR. Neuropathic pain associated with COVID-19: a systematic review of case reports. Curr Pain Headache Rep. 2022;26(8):1–9.
Kalangara JP, Galor A, Levitt RC, Felix ER, Alegret R, Sarantopoulos CD. Burning eye syndrome: do neuropathic pain mechanisms underlie chronic dry eye? Pain Med. 2016;17(4):746–55.
Kommoss FK, Schwab C, Tavernar L, Schreck J, Wagner WL, Merle U, Jonigk D, Schirmacher P, Longerich T. The pathology of severe COVID-19-related lung damage: mechanistic and therapeutic implications. Dtsch Arztebl Int. 2020;117(29–30):500.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 2008;9(1):1–13.
Lauria G, Majorana A, Borgna M, Lombardi R, Penza P, Padovani A, Sapelli P. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain. 2005;115(3):332–7.
Leite VF, Rampim DB, Jorge VC, de Lima MDCC, Cezarino LG, da Rocha CN, Esper RB. Prevent Senior COVID-19. Rehabilitation study persistent symptoms and disability after COVID-19 hospitalization: data from a comprehensive telerehabilitation program. Arch Phys Med Rehabil. 2021;102(7):1308–16. https://doi.org/10.1016/j.apmr.2021.03.001. (Epub 2021 Mar 10. PMID: 33711279; PMCID: PMC7943375).
Liampas A, Velidakis N, Georgiou T, Vadalouca A, Varrassi G, Hadjigeorgiou GM, Tsivgoulis G, Zis P. Prevalence and management challenges in central post-stroke neuropathic pain: a systematic review and meta-analysis. Adv Ther. 2020;37(7):3278–91.
Meduri A, Oliverio GW, Mancuso G, Giuffrida A, Guarneri C, Venanzi Rullo E, Nunnari G, Aragona P. Ocular surface manifestation of COVID-19 and tear film analysis. Sci Rep. 2020;10(1):1–7.
Muthyam AK, Reddy MP, Kulkarni S, Srilatha A, Sahithi K, Satyanarayana D. Oral manifestations in COVID-19 patients: an observational study. J Fam Med Prim Care. 2022;11(3):1000.
Niedźwiedź A, Kawa M, Pius-Sadowska E, Kuligowska A, Ziontkowska A, Wrzałek D, Parczewski M, Safranow K, Kozłowski K, Machaliński B, Machalińska A. Evaluating ocular symptoms and tear film cytokine profiles in symptomatic COVID-19 patients. J Clin Med. 2022;11(9):2647.
Patel SJ, Lundy DC. Ocular manifestations of autoimmune disease. Am Fam Physician. 2002;66(6):991.
Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123(3):A12–3.
Rokohl AC, Loreck N, Matos PAW, Zwingelberg S, Augustin M, Dewald F, Grajewski RS, Klein F, Lehmann C, Heindl LM. More than loss of taste and smell: burning watering eyes in coronavirus disease 2019. Clin Microbiol Infect. 2020;26(11):1560-e5.
Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: Physiopathology and treatment. World J Diabetes. 2015;6(3):432–44.
Seghal G, Bal P, Bal B, Chopra R. Pattern of ocular manifestations and the prevalence of severe acute respiratory syndrome coronavirus-2 in tears of hospitalized coronavirus disease 2019 patients. Taiwan J Ophthalmol. 2021;11(4):380.
Shaikh N, Al Mahdi H, Pai A, Pathare A, Abujaber AA, Dsliva A, Al-Jabry M, Subramanian K, Thomas S, Mohmed AS, Anjum S. Ocular manifestations of COVID-19: facts and figures from a tertiary care center. Ann Med. 2022;54(1):310–3.
Shouman K, Vanichkachorn G, Cheshire WP, Suarez MD, Shelly S, Lamotte GJ, Sandroni P, Benarroch EE, Berini SE, Cutsforth-Gregory JK, Coon EA. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31(3):385–94.
Sinjari B, D’Ardes D, Santilli M, Rexhepi I, D’Addazio G, Di Carlo P, Chiacchiaretta P, Caputi S, Cipollone F. SARS-CoV-2 and oral manifestation: an observational, human study. J Clin Med. 2020;9(10):3218.
Skrypnikova T, Skrypnykov P, Shynkevych V. Long term oral symptoms systematization in patients who underwent COVID-19: case series research. J Int Dent Med Res. 2022;15:1133–42.
Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32(1):19–41.
Sugiyama A, Miwata K, Kitahara Y, Okimoto M, Abe K, Ouoba S, Akita T, Tanimine N, Ohdan H, Kubo T, Nagasawa A. Long COVID occurrence in COVID-19 survivors. Sci Rep. 2022;12(1):1–11.
Vallejo-Garcia JL, Balia L, Raimondi R, Rustioni G, Camesasca FI, Borgia A, Fossati G, Confalonieri F, Legrottaglie EF, Casari E, Sandri MT. Conjunctivitis as a sign of persistent SARS-COV-2 infection? An observational study and report of late symptoms. Eur J Ophthalmol. 2022;32(2):830–5.
Villarroel-Dorrego M, Chacón L, Rosas R, Barrios V, Pernía Y, Vélez H. [Translated article] Oral findings in patients with COVID-19. Actas dermo-sifiliograficas. 2022;113(2):T183–6.
Fernández-de-Las-Peñas C, Notarte KI, Peligro PJ, Velasco JV, Ocampo MJ, Henry BM, Arendt-Nielsen L, Torres-Macho J, Plaza-Manzano G. Long-COVID symptoms in individuals infected with different SARS-CoV-2 variants of concern: a systematic review of the literature. Viruses. 2022;14(12):2629.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
L.D.W. screened the papers, collected the data, and drafted the manuscript. P.Z. designed the study, screened the papers, analyzed the data, and drafted the manuscript.
Disclosures
The authors have nothing to disclose.
Compliance with Ethics Guidelines
This systematic review and meta-analysis is based on published papers and there are no ethical issues.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Williams, L.D., Zis, P. COVID-19-Related Burning Eye Syndrome and Burning Mouth Syndrome: A Systematic Review and Meta-analysis. Pain Ther 12, 621–630 (2023). https://doi.org/10.1007/s40122-023-00492-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00492-3